Abstract
Changes gait parameters induced by the concomitant performance of one of two cognitive tasks activating working memory and spatial attention, was examined in healthy young adults (YA) and older adults (OA). There was a main effect of task condition on gait-speed (p= 0.02), stride-length (p<0.001) and double-support time (p=0.04) independent of the group. There were no significant differences between working memory and spatial attention associated gait changes. Working-memory and spatial-attention dual-tasking led to a decrease in gait-speed (p=0.09 and 0.01) and stride-length (p=0.04 and 0.01) and increase in double-support time (p=0.01 and 0.03) in YA and decrease in stride-length (p=0.04 and 0.01) alone in OA. Cognitive task associated changes in gait may be a function of limited attentional resources irrespective of the type of cognitive task.
Introduction
Dual-tasking studies on balance control and gait have enhanced our understanding of the influences of cognition on these functions (reviewed by Woollacott and Shumway-Cook(Woollacott and Shumway-Cook, 2002)). Dual-tasking methodology involves the performance of secondary tasks while walking to determine the costs involved in performing the concurrent task (Beauchet et al., 2002; Beauchet et al., 2003; Bowen et al., 2001; Camicioli et al., 1997; de Hoon et al., 2003; Ebersbach et al., 1995; Grabiner et al., 2001; Lundin-Olsson et al., 1997; O'Shea et al., 2002; Rushworth et al., 2001)(see methodology review by Huang and Mercer (Huang and Mercer, 2001)). Costs of dual-tasking on gait parameters are observed by studying changes in speed, cadence, step-length and double support time while performing secondary tasks; the decrements in gait parameters are presumed to be due to a limited attentional capacity depending on the complexity of the secondary task(Huang and Mercer, 2001; Woollacott and Shumway-Cook, 2002).
Various secondary tasks have been used to demonstrate the interactions between cognition and gait. Most studies have used speech as the distraction task (Beauchet et al., 2005; Beauchet et al., 2002; Beauchet et al., 2003; Bootsma-van der Wiel et al., 2003; Bowen et al., 2001; Camicioli et al., 1997; Camicioli et al., 1998b; Cocchini et al., 2004; Condron and Hill, 2002; de Hoon et al., 2003; Ebersbach et al., 1995; Haggard et al., 2000; Hauer et al., 2003; Huxhold et al., 2006; Kemper et al., 2003; Lundin-Olsson et al., 1997; Sheridan et al., 2003; van Iersel et al., 2007; Williams et al., 2006) where as others have used manual motor tasks(Bond and Morris, 2000; Lundin-Olsson et al., 1998; O'Shea et al., 2002; Toulotte et al., 2006) or even electrical stimulation as the secondary tasks(Regnaux et al., 2005). Interference effects of the secondary tasks depend on the study sample and complexity of the secondary task. For example, effects on gait parameters were observed on a counting-backwards task in older adults but not in young adults (Beauchet et al.) and on a digit span task in patients with Alzheimer's Disease (Sheridan et al., 2003) but not in young adults (Ebersbach et al., 1995). Some studies suggest that the respiratory alterations associated with speech production and/or central interference between regions involved in motor control as well as articulation or the rhythmic components of speech may play a role in dual-task interference rather than the competing demands on attention. Ebersach et al. studied the effect on gait with concurrent secondary tasks including a digit span task, opening and closing buttons task and finger tapping and found that stride time decreased with concurrent finger tapping and double support time increased only when digit span was performed along with opening and closing buttons while walking; digit span or the button task independently had no effect on gait parameters(Ebersbach et al., 1995). Similarly, reaction time tasks in response to an auditory stimuli did not affect gait parameters but reciprocal effects of walking were seen on reaction time(Lajoie et al., 1993; Sparrow et al., 2002). These studies suggest that the interference effect of a secondary-task on gait may depend on the type of secondary task, which may relate to whether or not the two concurrent processes share common neuronal resources (Huang and Mercer, 2001). For example, some secondary-tasks such as listening have no effect on gait parameters when performed concurrently(Lajoie et al., 1993; Lajoie et al., 1996)
Executive function refers to the ability to conceptualize, abstract, organize, initiate and regulate complex behaviour (Stuss and Benson, 1986) and comprises higher-level functions such as attentional capacity and working memory (the ability to mentally manipulate information). Dual-task studies indicate that executive function tasks influence gait performance in community-dwelling older adults(Coppin et al., 2006; Holtzer et al., 2006; Kuo et al., 2007; Springer et al., 2006). For example, arithmetic tasks but not semantic fluency (generating a list of animals) are more likely to lead to alterations in gait under dual-task conditions in older adults (Beauchet et al., 2005). Similarly, executive function tasks have also shown to alter gait parameters in patients with Alzheimer's Disease (Sheridan et al.) and Parkinson's Disease (Camicioli et al.). Working memory is an executive function requiring transient maintenance and concurrent manipulation of information for a goal-directed activity, which is utilized in routine daily activites(Baddeley, 1992). Spatial attention refers to the ability to shift the focus of awareness from one spatial location to the another (Posner, 1980). Spatial attention and working memory share common cognitive features (dynamic shifting of attentional resources) as well as few common brain activations on functional neuroimaging (supplementary motor areas and intra-parietal sulcus)(LaBar et al., 1999); however, these tasks differ in that the former is primarily a task of visuo-spatial attention associated with predominantly posterior brain regions whereas the latter is an executive-function task associated with predominantly anterior brain regions (Mesulam, 1998). It is unclear whether these two different cognitive tasks independently interfere with walking if performed simultaneously while walking.
The goal of this study was to determine whether these two cognitive tasks under dual-task conditions would lead to comparable changes in gait parameters in healthy young adults. The degree to which mental tasks interfere with walking increase with age suggesting that aging is associated with a greater demand on attentional resources required for efficiently carrying out both tasks(Chen et al., 1996; Lajoie et al., 1996; Lindenberger et al., 2000; Maki et al., 2001). Therefore, we also studied a smaller sample of healthy older adults to compare to that of the healthy young adults. We hypothesized that performance of cognitive tasks would lead to a decrease in gait speed, stride-length and double-support to provide a more stable gait pattern during dual-tasking and these dual-task related changes in gait would be larger in the older adult group compared to younger adult group. We also hypothesized that the changes in gait parameters during dual-tasking would depend on the attentional load of the secondary task such that under dual-task conditions, our working memory task, which was designed to be cognitively more demanding than the spatial attention task, would lead to larger effects on gait parameters than the spatial attention task.
Methods
Participants
Ten young adults (mean age: 27 years) and 10 older adults (mean age: 75 years). The older adults were recruited from a community-dwelling pool of healthy elders participating in the Sunnybrook Dementia Study, a longitudinal study with annual neuropsychological testing, neuroimaging and functional assessments. Cognitive impairment, gait impairment or any condition that interfered with gait were exclusionary. The older adult participants were within normal limits on detailed neuropsychological testing. The study was conducted in a gait laboratory of a university hospital with approval from the Institutional Research Ethics Board.
Apparatus
Gait parameters were measured using GaitRite® (CIR systems, Inc., Havertown, PA), a computerized walkway that records the temporal and spatial parameters of each participant's gait for subsequent analysis. It contains a grid of pressure-activated sensors that are encapsulated in a carpeted walkway measuring 12 × 2 feet. The accompanying software (GAITRite Gold, Version 3.2b) reconstructs each traverse across the walkway and automatically computes the spatial and temporal parameters for every traverse.
The stimuli for the cognitive paradigms were presented using Labview® software (National Instruments Corporation, Austin, TX). The stimuli were projected on a screen placed at either end of the walkway in the direct view of the participant's central gaze as they walked on the walkway (Figure 1a). Accuracy of cognitive tasks was captured by means of a small hand-held button device connected to the computer via an analogue-to-digital converter. The data were acquired and analyzed through Labview software. The sampling frequency was set at 500Hz.
Figure 1. Figure 1a: Experimental set-up.
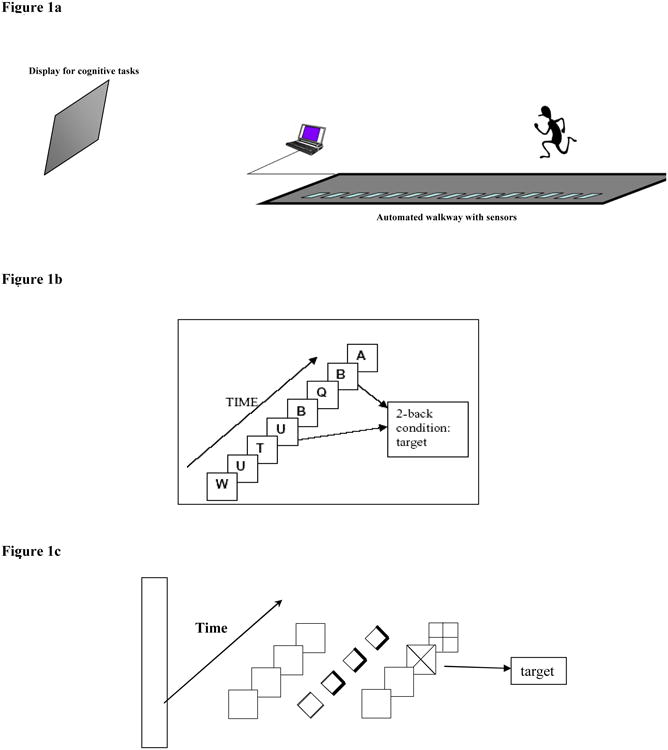
Figure 1b: Working memory task ( 2-back task):
Figure 1c: Spatial attention task:
Gait
Gait-speed, stride-length and double-support were measured at the participants' preferred-pace and captured during a steady-state gait. To ensure this, we instructed participants to start walking approximately 3 feet prior to stepping on the walkway and continue walking up to 3 feet beyond the end of the computerized walkway. Gait parameters were measured across three conditions: walking only, without concomitant cognitive tasking, or walking while performing the working memory task and walking while performing the spatial attention task. Each condition comprised five traverses across the walkway. For collecting gait data on dual-tasking, cognitive paradigms were introduced while they were standing and participants were prompted to begin walking after they were engaged in the cognitive task for at least 10 seconds.
Cognitive tasks
Letter 2-back working memory task (Figure 1b)
The working memory task was a verbal 2-back task(Gevins and Cutillo, 1993). In every trial, participants were shown a continuous stream of letters that were flashed on a screen. Participants were to respond by pressing the button if a presented letter was the same as the one that came up two stimuli back in the sequence. This task had a high working memory load as it required the continuous maintenance of each stimulus in memory until two consecutive stimuli appeared and required continuous on-line monitoring of the presented stimulus in order to execute the response as soon as the stimulus matched the one that came up two stimuli prior to it. This task did not require shifts of spatial-attention as the letters appear continuously in the centre of the screen.
Spatial attention task (Figure 1c)
The spatial attention task(Posner and Dehaene, 1994) examined covert shifts of spatial attention. Participants maintained fixation on a central point on the screen where an arrow appeared pointing to the left or right. Immediately, a stimulus appeared in one of the two peripheral boxes located on either side of the centrally placed arrows. The stimuli were of two types either a “X ”or a “2”. The participants were instructed to respond to the target, an “X” only [not a “2”] by pressing the button on the hand-held device as rapidly as possible. The central cue remained visible until the stimulus appeared on the periphery triggering a covert shift of attention to the peripheral stimuli.
Study design
Participants were tested individually during a single session. At the start of the session all participants received detailed instructions on how to perform the cognitive conditions. Every participant practiced the cognitive conditions prior to testing sitting in front of a computer screen in order to achieve an accuracy of 100% while performing the tasks. For analysis, session included a single-task walking condition, two single-task cognitive conditions (verbal 2-back working memory or a covert spatial attention task) and two dual-task conditions (walking plus either one of the two cognitive tasks). The single-task cognitive conditions consisted of five trials of 60 second duration each (detailed below). The single-task walking condition consisted of five traverses across the walkway with a button device held in the dominant hand and with gaze fixated at a mark centered on the screen across the walkway. The dual-task conditions also comprised five trials of walking across the walkway while performing the two cognitive tasks described below, one at a time in succession. Participants registered their responses on the cognitive tasks by pressing a hand-held button held in their dominant hand. The display duration for every stimulus on the cognitive paradigms was set to 500ms with an inter-stimulus interval of 1500 seconds thereby allowing for approximately 7-10 stimuli for every trial. Participants were encouraged to perform the task to the best of their ability. i.e continue to aim at 100% accuracy but also register their responses as quickly as possible. In order to assess gait changes during optimal cognitive performance, any dual-task trial with a drop in accuracy of 10% or more was excluded from data collection. The order of condition was randomized but participants were informed about the task condition prior to every traverse across the walkway.
Statistical Analysis
The data were analyzed using a 2×3 repeated measures analysis of variance (ANOVA) with age group (YA vs OA) as a between-subjects factor and task condition as within-subjects factor (no cognitive task vs working-memory vs spatial-attention). Changes in gait parameters (speed, stride-length and double-support) while dual-tasking were compared in the two groups. Partial eta squared values (ηP2) were obtained as measures of effect size. To identify significant differences between changes in gait parameters between the two cognitive dual-task conditions within each group, pairwise t-tests were used.. Performance on cognitive tasks was not analyzed for this report.
Results
Baseline characteristics
Demographic and baseline walking conditions in the two groups are highlighted in table 1. Besides age and weight, there were no significant differences between the two groups on demographic variables such as height, gender and leg length. There were no differences in baseline walking condition (walking without cognitive tasking) between the two groups on gait speed, stride-length, cadence, and double-support.
Table 1. Baseline differences in young and older adult groups.
| YA (n=10) | OA (n=10) | P value | |
|---|---|---|---|
| Age | 27.3±4 | 74.3±7 | <0.001 |
| Weight | 59±6 | 71±15 | 0.05 |
| Height | 1.63±0.05 | 1.62±0.09 | 0.9 |
| Leg length | 0.84±0.04 | 0.79±0.2 | 0.4 |
| Gender (N of female) | 5 | 7 | 0.65 |
| Gait speed (m/sec) | 1.22±0.1 | 1.20±0.2 | 0.7 |
| Stride-length (m) | 1.31±0.07 | 1.32±0.2 | 0.9 |
| Cadence (steps/min) | 113±6 | 109±7 | 0.3 |
| Double-support (sec) | 0.26±0.04 | 0.29±0.1 | 0.9 |
Dual-task effect on gait parameters
Speed (Figure2, Table 2)
Figure 2.
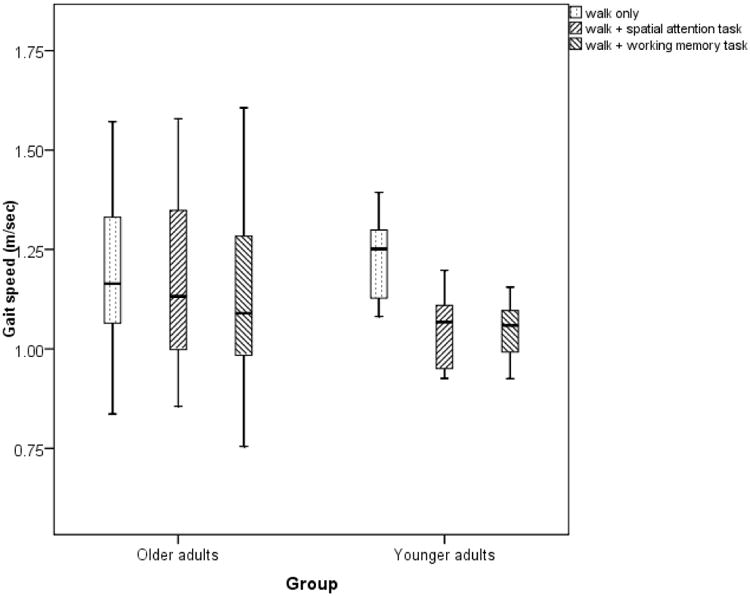
Changes in speed in young adults and older adults with dual-tasking.
Table 2.
Changes in regular paced gait parameters with concurrent spatial-attention and working-memory task performance in young adult and older adult groups (p values indicate level of significance in differences between respective dual-task conditions as compared to regular paced gait parameters).
| 2a. Young adults: | |||||
|---|---|---|---|---|---|
| Regular paced walking (mean±SD) | Dual-tasking, i.e, walking + | ||||
| Spatial-attention (mean±SD/p value) | Working memory (mean±SD/p value) | ||||
| Speed (m/sec) | 1.22±0.1 | 1.06±0.1 P=0.003 |
1.05±0.1 P=0.002 |
||
| Stride-length (m) | 1.31±0.1 | 1.21±0.1 P=0.006 |
1.21±0.1 P=0.003 |
||
| Cadence (steps/min) | 112.5±6.4 | 105.4±4.7 P=0.007 |
104.7±5.6 P=0.004 |
||
| 2b. Older adults: | ||||||
|---|---|---|---|---|---|---|
| Regular Paced walking (mean±SD) | Dual-tasking, i.e, walking + | |||||
| Spatial-attention (mean±SD/p value) | Working memory (mean±SD/p value) | |||||
| Speed (m/sec) | 1.20±0.22 | 1.17±0.23 P=0.2 |
1.16±0.27 P=0.3 |
|||
| Stride-length (m) | 1.33±0.2 | 1.27±0.21 P=0.01 |
1.26±0.24 P=0.04 |
|||
| Cadence (steps/min) | 109.4±7.3 | 110.9±9.1 P=0.3 |
110.2±7.6 P=0.7 |
|||
There was a main effect of dual-task condition on speed (F(2,18)=13.05, p=0.002, ηP2 = 0.42). There was a significant task × age-group interaction within the groups (F(2,18)=5.6, p=0.03, ηP2 = 0.24) and the differences between-groups were significant (F(2,18)=0.8, p=0.3, ηP2 = 0.04). Gait-speed decreased significantly while performing spatial-attention tasks (0.17 ± 0.13 m/sec, p=0.003) and working memory tasks (0.17±0.13 m/sec, p=0.002) in the YA group. In the OA group gait speed decreased with concomitant spatial-attention tasking (0.03 ± 0.08 m/sec, p=0.2) and working-memory (0.043 ± 0.13 m/sec, p=0.3) but there were no statistical significant differences between the two tasks.
Stride-length (Figure 3, Table 2)
Figure 3. Changes in stride-length in young adults and older adults with dual-tasking.
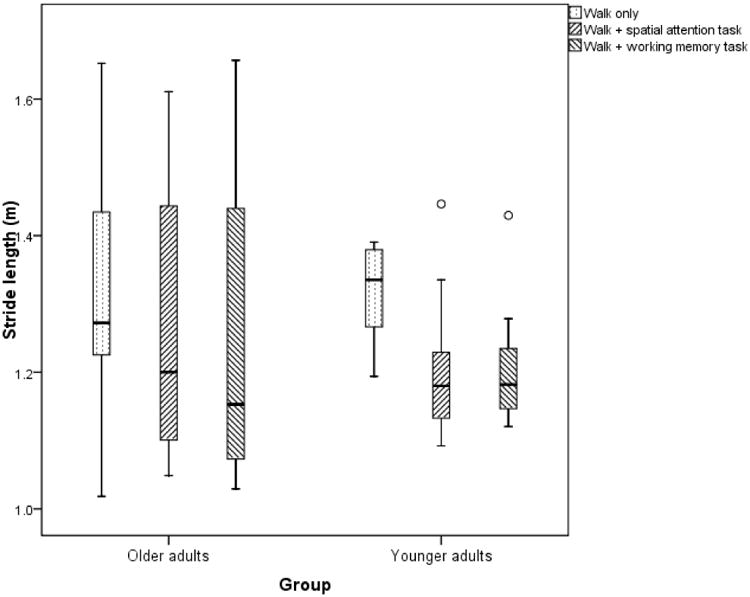
There was a main effect of dual-task condition on stride-length (F(1,36)= 10.032, p<0.01, ηP2 = 0.2). There was no significant task × age-group interaction within groups (F(2,18)=2, p=0.17, ηP2 = 0.1) and there were no significant between-group differences as well (F(2,18)=0.4, p=0.5, ηP2 = 0.02). The decrease in stride-length with concurrent spatial-attention (0.107 ± 0.09 m, p=0.006) and working-memory task (0.106 ± 0.08 m, p=0.003) were significant in YA group. The decrease in stride-length in the OA group was also significant during concurrent spatial-attention (0.05 ± 0.05 m, p=0.01) and working-memory (0.06 ± 0.08 m, p=0.04) tasks. There were no statistically significant differences in stride-length between working-memory and spatial attention conditions in the two groups.
Cadence (Figure 4, Table 1)
Figure 4.
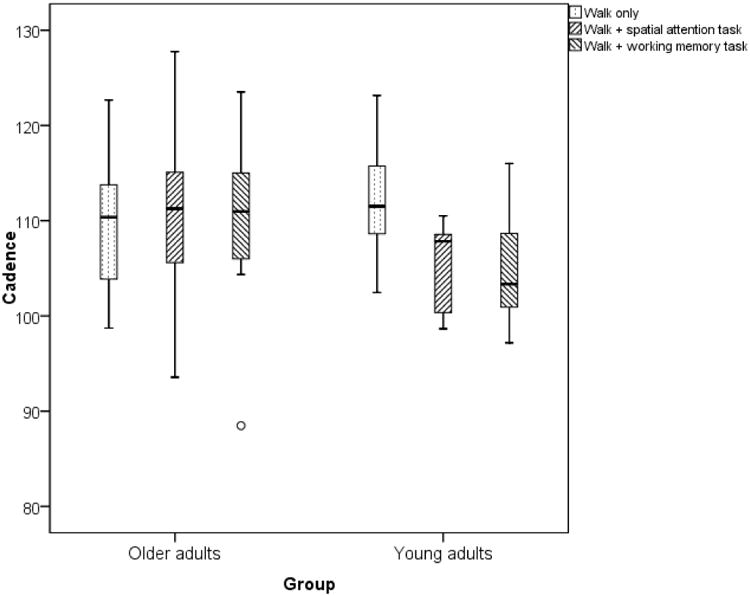
Changes in cadence in young adults and older adults with dual-tasking.
There was a main effect of dual-task condition on cadence (F(2,18)=4.6, p=0.04, ηP2 = 0.26). There was a significant task × age-group interaction within-groups (F(2,18)=8.2, p=0.01, ηP2 = 0.3) but not between groups (F(2,18)=0.8, p=0.3, ηP2 = 0.4). In the YA group, the changes in cadence with spatial attention task (7.1±6.5, p=0.007) and working memory task (7.8±6.4, p=0.004) were statistically significant but again there was no significant change in cadence between the two conditions (p=0.6). In the OA group, there was no significant change in cadence during spatial-attention task (1.04 ±4.9, p=0.4) and working-memory task (0.78±6.7, p=0.7).
Gait stability ratio (GSR=steps per meter)
(Figure 5)The ratio of cadence to speed has been suggested to better estimate gait stability than gait speed{Cromwell, 2004 #1497}. When the GSR was compared between the two groups there was a main effect of GSR ((F(2,18)=17, p=0.001, ηP2 = 0.48) but no interaction between the condition and group. There were no significant within or between group differences on this derived variable.
Figure 5.
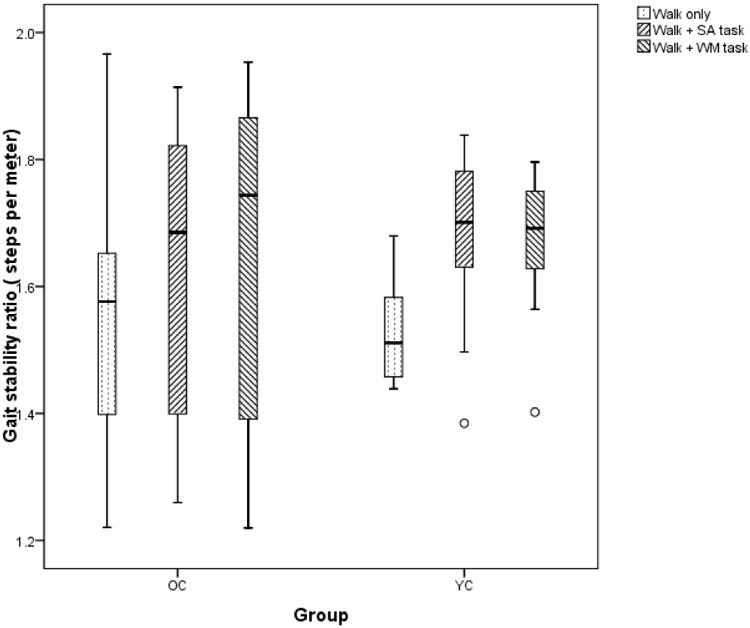
Gait stability ratio (GSR= ratio of cadence to speed or steps per meter) in the two groups.
Discussion
Results of this study support the hypothesis that changes in gait parameters occur with concomitant performance of cognitive tasks in older and younger adults. However, the strategies were different in both groups. When young adults are engaged in performing spatial-attention and working memory tasks while walking their gait speed is significantly slower and their stride length also significantly reduces. The older adults, on the other hand, decrease their stride-length but maintain their gait speed at the cost of increasing their cadence. The differences in dual-task gait parameters between the two cognitive tasks were not significant. These results support the hypothesis that both groups adapted their gait patterns while cognitive tasking, but do not support the hypothesis that changes on tasks expected to more cognitively demanding, showed a trend towards greater magnitude of changes in gait. The changes in dual-tasking in older adults were not significantly different from the changes in dual-tasking in the young adult group.
The changes in gait speed observed in this sample of young adults are consistent with other studies showing that gait speed decreases on performing a secondary task {Beauchet, 2002 #935}{Springer, 2006 #1360}(Ebersbach et al., 1995). A few studies have also shown that cadence increases on dual-tasking similar to the findings in our sample of older adults{Beauchet, 2005 #1361}. The results of this study suggest that young and old adults maintain their gait stability while dual-tasking with no significant differences between the two groups on the gait stability ratio (GSR). Therefore, young and older adults may achieve the same result of maintaining gait stability either by decreasing the speed as we saw in the young adults or by increasing the cadence as observed in the older adults. Both maneuvers provide a decrease in stride-length as a adaptive mechanism while dual-tasking. The current study also extends previous knowledge by demonstrating that concurrent cognitive activity alone can influence gait parameters that is, without interference effects from concurrent manual or speech activity which is noteworthy as previous studies have also shown that postural stability may be directly influenced by speech production while performing word generation tasks, and talking while walking or repetition of digits(Yardley et al., 1999).
We found that the changes in gait parameters were similar for both working memory and spatial-attention tasks. The changes were more marked during the working memory task but there were no statistical significant differences on costs of gait parameters between the two tasks. The lack of difference in between the two cognitive tasks could relate to commonality between the two tasks that is both tasks are attention demanding task and that the change in gait parameters may relate more to the sharing of attentional resources rather than the cognitive mode of gait interference. It is postulated that under dual-task situations, resource sharing of common neuronal areas that sub-serve individual tasks involved may lead to “capacity-sharing” and/or “bottle-necking” of common resources, leading to decrements in both tasks (Pashler, 1994). The interference effects for different concurrent motor or cognitive tasks may then depend on whether or not these concurrent processes compete for the same neuronal resources(Huang and Mercer, 2001). Functional MRI studies of working memory and spatial attention tasks have revealed that these tasks evoke a network of activations in multiple frontoparietal regions such as the supplementary motor area, banks of the intraparietal sulcus, striatum and cerebellar vermis (LaBar et al., 1999). Functional neuroimaging studies have also suggested that these regions may play an important role in human locomotion(Fukuyama et al., 1997; Malouin et al., 2003; Miyai et al., 2001). The premotor and prefrontal regions appear to be involved in the maintenance of an individual's walking pace(Suzuki et al., 2004), while areas within the parietal lobe such as the banks of the intraparietal sulcus may play a role in informing about relative positions of body parts and modulating limb movements (Rushworth et al., 2003). Therefore, working memory and spatial attention may also share in part the neuronal resources that control gait speed and other temporal parameters, which may explain why no significant differences were seen between the two dual-tasks in this study.
This methodology in this study differs from other dual-task studies on gait in that the secondary tasks used in this study targeted unitary cognitive functions, namely working memory and spatial attention. The paradigms were designed to minimize interference by other concurrent cognitive processes and limit motor interference to only a button-press in the dominant hand. We used sensitive gait assessment devices in capturing specific gait parameters in two groups of healthy young and older adults. This study has its limitations as well. Firstly, the advantage of using an automated walkway to enable accurate and easy capture of gait parameters was compromised by the relatively short length of the walkway (12 feet), as we were unable to capture continuous gait parameters beyond the duration required to complete a single traverse. To mitigate this drawback we averaged gait parameters over 5 traverses for each condition. Secondly, there was a significant main effect of dual-task condition on gait parameters in the two groups but the effect sizes (denoted by partial eta squared values (ηP2)) for the main effects were small (in range of 0.1 to 0.2). The small effect size in this study may reflect the fact that the older adult sample was relatively smaller and sensitive automated gait assessment systems used in this study. Smaller effect sizes could also be due to small sample sizes of our groups. Effect sizes are not usually reported in dual-task gait studies in healthy individuals and comparisons with those targeting gait-impaired populations cannot be made. Lastly, though the paradigms were programmed to capture reaction times during performance of cognitive tasks, inspection of reaction time data collected showed that these were incorrectly registered upon capture and hence reaction time data could not be reliably analyzable for this report. However, the hypotheses that gait changes occur while cognitive dual-tasking at optimal accuracy was justifiable despite the loss of the reaction time data.
In summary, a concurrent working-memory and spatial-attention task performed while walking in healthy young and older adults led to a changes in gait in both groups with each group adopting different strategies with a resulting common goal of maintaining stability. There was a trend for increased costs of working-memory task performance on gait parameters in comparison to spatial-attention task performance in both groups. Whether the change in temporal gait parameters is an innate compensatory response to increase stability of gait while dual-tasking, or results from competition of the concurrent processes for common neuronal resources, needs to be further investigated. The dual task used in this study can be used to elucidate possible interactions between working memory and gait control in pathological conditions associated with compromised neuronal resources such as in neurodegenerative and cerebrovascular disease.
References
- Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? J Mot Behav. 2005;37:259–64. [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Stierlam F, Blanchon MA, Mourey F, Pfitzenmeyer P, et al. Influence of a specific cognitive task on spatial-temporal walking parameters in elderly frail individuals. Presse Med. 2002;31:1117–22. [PubMed] [Google Scholar]
- Beauchet O, Kressig RW, Najafi B, Aminian K, Dubost V, Mourey F. Age-related decline of gait control under a dual-task condition. J Am Geriatr Soc. 2003;51:1187–8. doi: 10.1046/j.1532-5415.2003.51385.x. [DOI] [PubMed] [Google Scholar]
- Bond JM, Morris M. Goal-directed secondary motor tasks: their effects on gait in subjects with Parkinson disease. Arch Phys Med Rehabil. 2000;81:110–6. doi: 10.1016/s0003-9993(00)90230-2. [DOI] [PubMed] [Google Scholar]
- Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, van Exel E, Bloem BR, Westendorp RG. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1466–71. doi: 10.1046/j.1532-5415.2003.51468.x. [DOI] [PubMed] [Google Scholar]
- Bowen A, Wenman R, Mickelborough J, Foster J, Hill E, Tallis R. Dual-task effects of talking while walking on speed and balance following a stroke. Age Ageing. 2001;30:319–23. doi: 10.1093/ageing/30.4.319. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48:955–8. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998a;50:1496–8. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Oken BS, Sexton G, Kaye JA, Nutt JG. Verbal fluency task affects gait in Parkinson's disease with motor freezing. J Geriatr Psychiatry Neurol. 1998b;11:181–5. doi: 10.1177/089198879901100403. [DOI] [PubMed] [Google Scholar]
- Chen HC, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire KE. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol A Biol Sci Med Sci. 1996;51:M116–22. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- Cocchini G, Della Sala S, Logie RH, Pagani R, Sacco L, Spinnler H. Dual task effects of walking when talking in Alzheimer's disease. Rev Neurol (Paris) 2004;160:74–80. [PubMed] [Google Scholar]
- Condron JE, Hill KD. Reliability and validity of a dual-task force platform assessment of balance performance: effect of age, balance impairment, and cognitive task. J Am Geriatr Soc. 2002;50:157–62. doi: 10.1046/j.1532-5415.2002.50022.x. [DOI] [PubMed] [Google Scholar]
- Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–24. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dault MC, Yardley L, Frank JS. Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res Cogn Brain Res. 2003;16:434–40. doi: 10.1016/s0926-6410(03)00058-2. [DOI] [PubMed] [Google Scholar]
- de Hoon EW, Allum JH, Carpenter MG, Salis C, Bloem BR, Conzelmann M, et al. Quantitative assessment of the stops walking while talking test in the elderly. Arch Phys Med Rehabil. 2003;84:838–42. doi: 10.1016/s0003-9993(02)04951-1. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Dimitrijevic MR, Poewe W. Influence of concurrent tasks on gait: a dual-task approach. Percept Mot Skills. 1995;81:107–13. doi: 10.2466/pms.1995.81.1.107. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–6. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–43. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Grabiner PC, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82:31–5. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry. 2000;69:479–486. doi: 10.1136/jnnp.69.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer K, Pfisterer M, Weber C, Wezler N, Kliegel M, Oster P. Cognitive impairment decreases postural control during dual tasks in geriatric patients with a history of severe falls. J Am Geriatr Soc. 2003;51:1638–44. doi: 10.1046/j.1532-5415.2003.51517.x. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait speed: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–23. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Huang H-J, Mercer VS. Dual-Task Methodology: Applications in Studies of Cognitive and Motor Performance in Adults and Children. Pediatric Physical Therapy. 2001;13:133–140. [PubMed] [Google Scholar]
- Huxhold O, Li SC, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kemper S, Herman RE, Lian CH. The costs of doing two things at once for young and older adults: talking while walking, finger tapping, and ignoring speech or noise. Psychol Aging. 2003;18:181–92. doi: 10.1037/0882-7974.18.2.181. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Leveille SG, Yu YH, Milberg WP. Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. Gerontology. 2007;53:102–10. doi: 10.1159/000096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res. 1993;97:139–44. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Upright standing and gait: are there changes in attentional requirements related to normal aging? Exp Aging Res. 1996;22:185–98. doi: 10.1080/03610739608254006. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–36. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. Stops walking when talking as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc. 1998;46:758–61. doi: 10.1111/j.1532-5415.1998.tb03813.x. [DOI] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–20. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Maki BE, Zecevic A, Bateni H, Kirshenbaum N, McIlroy WE. Cognitive demands of executing postural reactions: does aging impede attention switching? Neuroreport. 2001;12:3583–7. doi: 10.1097/00001756-200111160-00042. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–92. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- O'Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–97. [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–44. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–9. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Regnaux JP, David D, Daniel O, Smail DB, Combeaud M, Bussel B. Evidence for cognitive processes involved in the control of steady state of walking in healthy subjects and after cerebral damage. Neurorehabil Neural Repair. 2005;19:125–32. doi: 10.1177/1545968305275612. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4:656–61. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer's disease. J Am Geriatr Soc. 2003;51:1633–7. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- Sparrow WA, Bradshaw EJ, Lamoureux E, Tirosh O. Ageing effects on the attention demands of walking. Hum Mov Sci. 2002;21:961–72. doi: 10.1016/s0167-9457(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–7. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. New York: Raven Press; 1986. [Google Scholar]
- Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage. 2004;23:1020–6. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Toulotte C, Thevenon A, Watelain E, Fabre C. Identification of healthy elderly fallers and non-fallers by gait analysis under dual-task conditions. Clin Rehabil. 2006;20:269–76. doi: 10.1191/0269215506cr929oa. [DOI] [PubMed] [Google Scholar]
- van Iersel MB, Ribbers H, Munneke M, Borm GF, Rikkert MG. The effect of cognitive dual tasks on balance during walking in physically fit elderly people. Arch Phys Med Rehabil. 2007;88:187–91. doi: 10.1016/j.apmr.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Williams K, Hinton VA, Bories T, Kovacs CR. Age and function differences in shared task performance: walking and talking. Res Q Exerc Sport. 2006;77:137–41. doi: 10.1080/02701367.2006.10599340. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Yardley L, Gardner M, Leadbetter A, Lavie N. Effect of articulatory and mental tasks on postural control. Neuroreport. 1999;10:215–9. doi: 10.1097/00001756-199902050-00003. [DOI] [PubMed] [Google Scholar]


