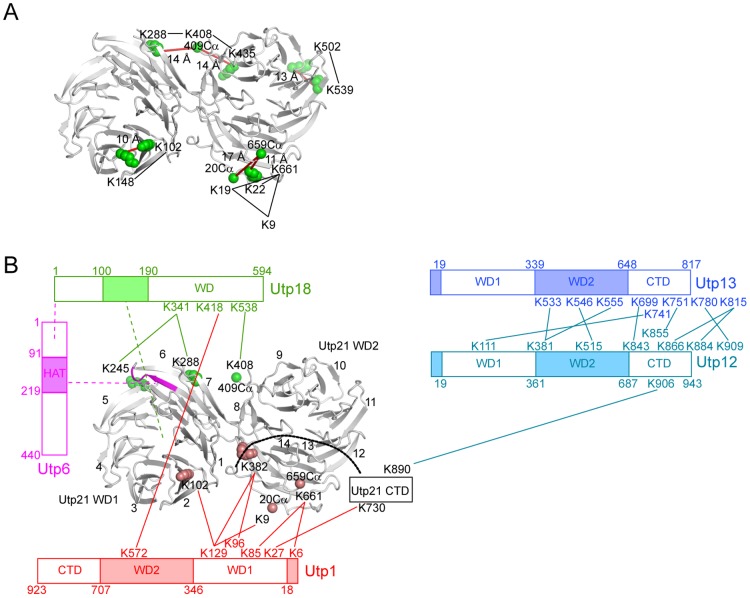
Figure 5. Organization of UTPB.
(A) Mapping of intramolecular crosslinking sites on the Utp21 tandem WD domain structure. Crosslinked lysines are shown as green spheres. When a crosslinked lysine (K9, K19, K408 and K661) is disordered in the crystal structure, the Cα atom of its closest terminal residue (residues 20, 409 and 659) is displayed as a sphere as a reference point. Crosslinked lysines are connected by black lines. The Cα-Cα distances between crosslinked lysines or reference points are indicated and marked as red lines. (B) Architecture of the UTPB complex. The intermolecular crosslinks are mapped to the Utp21 tandem WD domain structure or shown on the domain diagrams of other proteins. Lines denote crosslinks. Dashed lines denote interactions demonstrated by yeast 2-hybrid or pull-down assays. Residues crosslinked to Utp18 are colored in green, residues crosslinked to Utp1 are colored in red, and residues 274–279, the key binding site of the Utp6 HAT domain, are colored in magenta.