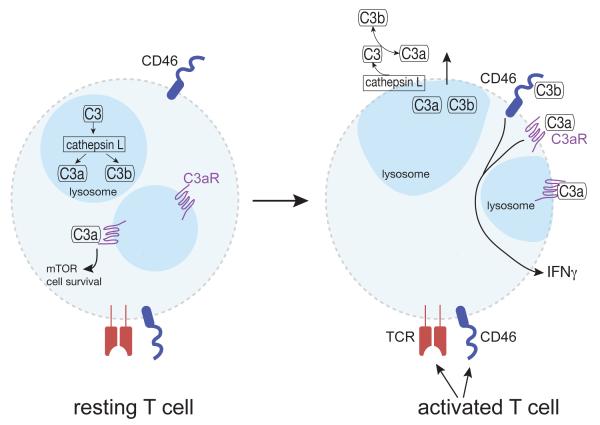
Figure 1. Cathepsin L-dependent intracellular and extracellular complement activation pathways.
In resting T cells, the complement factor C3 is contained intracellularly in performed stores and is proteolytically cleaved to C3a and C3b, by cathepsin L within lysosomes. Intracellularly generated C3a signals via engagement of intracellular C3aRs to promote cell survival via mTOR activation. Unstimulated T cells do not express surface C3, C3a, C3b, the C3aR or cathepsin L. However, upon engagement of the TCR alone or the TCR plus CD46, T cells release C3, C3a, C3b, as well as cathepsin L and express surface C3aR. On the cell surface, cathepsin L cleavesC3 to generate C3a and C3b. Subsequent binding of C3a to C3ar, and C3b to CD46 induces T cell secretion of cytokines such as IFN-γ and IL-17A.