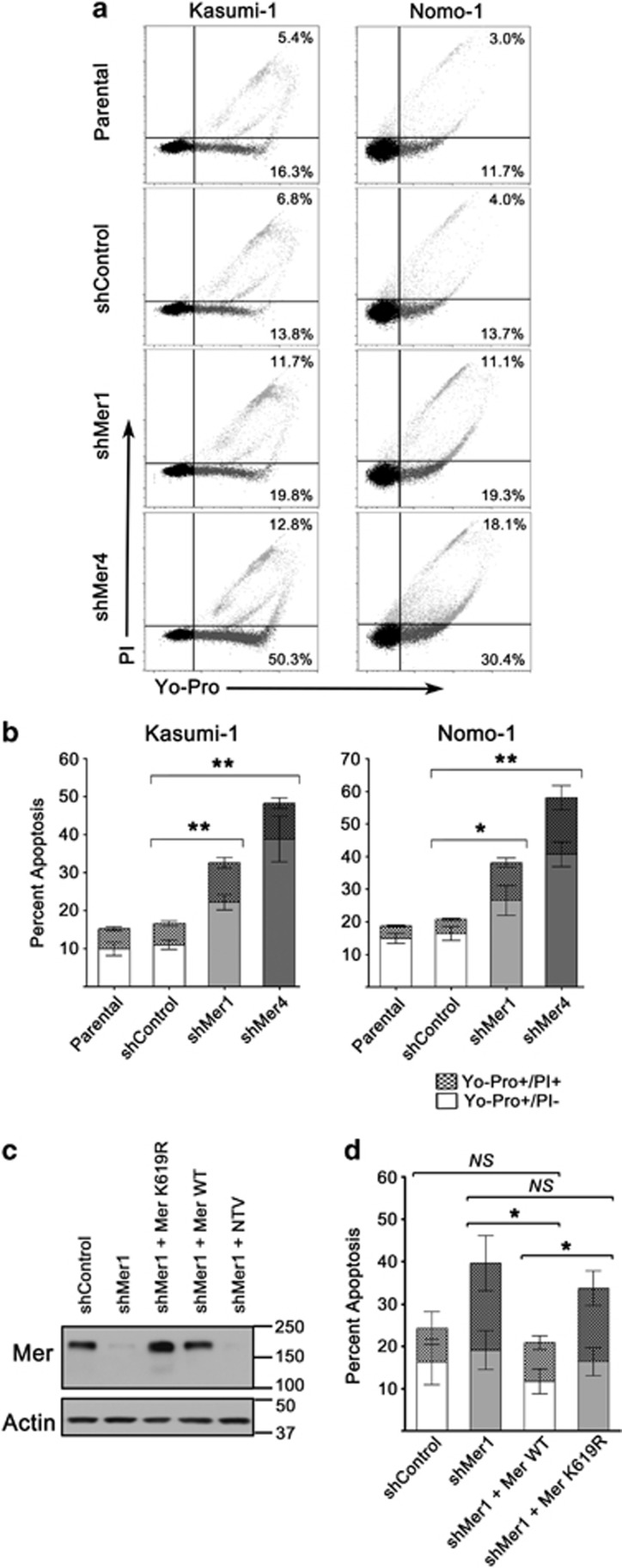
Figure 5.
Mer knockdown confers susceptibility to apoptosis. (a) Parental, non-silencing control shRNA (shControl) and Mer knockdown (shMer1, shMer4) Kasumi-1 and Nomo-1 cells were serum starved or cultured in complete medium for 18 h before staining with propidium iodide and Yo-Pro-1 iodide. Apoptotic cells were identified by flow cytometry. Representative flow cytometry profiles are shown. The percentages of early apoptotic (lower right quadrant; Yo-Pro+/PI−) and late apoptosis/necrosis (upper right quadrant; Yo-Pro+/PI+) cells are shown. (b) Cumulative data demonstrate a significant accumulation of apoptotic cells in Kasumi-1 and Nomo-1 cells when Mer is inhibited by shRNA knockdown (*P<0.05 and **P<0.01 versus shControl). No significant differences between parental and shControl cells were observed. (c) Reintroduction of WT Mer into Kasumi-1 shMer1 knockdown cells rescues Mer protein level after knockdown (shMer1+Mer WT). Additional control cell lines were generated by transduction of a gene for a kinase mutant Mer (shMer1+Mer K619R), which maintains the extracellular epitope that binds the anti-Mer antibody, or a non-targeting vector (shMer1+NTV) into Kasumi-1 shMer1 cells. Representative immunoblot of Mer protein levels shown here. (d) Apoptosis of Kasumi-1 WT add-back (shMer1+Mer WT) and mutant Mer add-back (shMer1+Mer K619R) cells were analyzed in comparison to shControl and shMer1 cells after serum starvation as described above. Mer WT add-back rescues myeloblast ability to stave off apoptosis comparable to shControl cell lines (NS=not significant compared to shControl, *P=0.05 compared with shMer1 and shMer1+K619R). Mean values and s.e. derived from at least three independent experiments are shown.