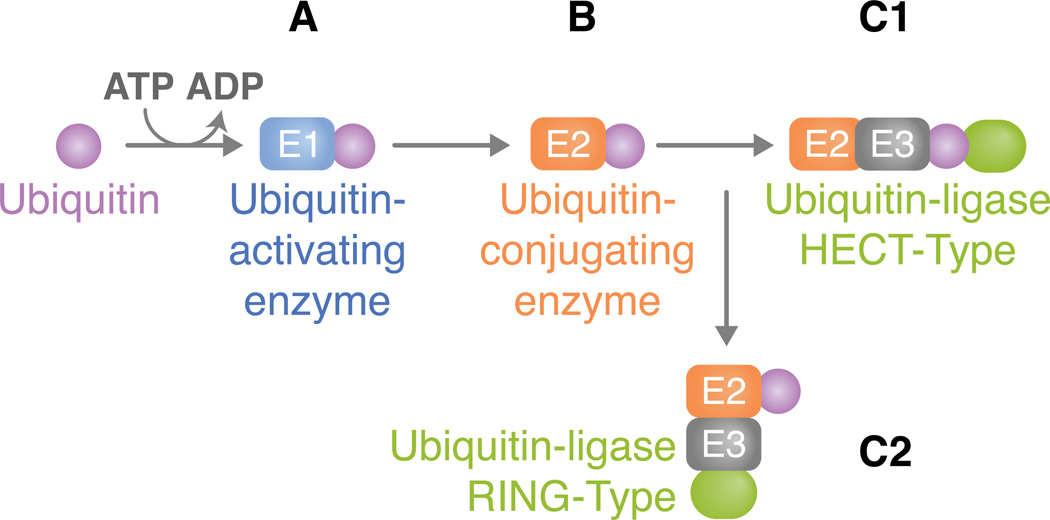
Figure 1. The ubiquitination pathway.
(A) For an ubiquitin to be added to a target lysine, a thiol ester bond between the E1 and ubiquitin is first formed in a two-step ATP-dependent reaction. Ubiquitin is activated through its combination with MgATP. This, in turn, forms a ubiquitin adenylate intermediate that acts as a ubiquitin donor to the E1 active site, creating an E1 molecule that consists of both an activated thiol ester ubiquitin and an adenylated ubiquitin. (B) The thiol-linked ubiquitin is then transferred to the E2. E2s typically share a core domain of about 150 amino acids and a cysteine residue in the active site to which E1 ubiquitin is transferred. (C) The ubiquitin ligase is typically a protein complex that attaches to both the substrate and the E2. The method in which substrate binding occurs varies between direct interactions or through auxiliary proteins. E3 enzymes can be classified according to the domains they carry. Here, we address two types of ubiquitin ligases: (C1) HECT type or (C2) RING type. (C1) HECT domain ligases have a domain that is approximately 350 residues. They serve a catalytic role in the conjugation of ubiquitin to the target protein (green oval). HECT domain proteins possess a conserved cysteine residue through which the activated E2 ubiquitin can form a thiol ester bond, thus leading to its transfer from E2. After attachment of ubiquitin, a lysine residue of the target protein must be introduced to the active site in order for substrate-ubiquitin ligation to occur. Ubiquitin is ultimately conjugated to a NH2 group of the target protein. The difference in the NH2 terminal domain within various HECT domain proteins is responsible for E3 substrate specificity. (C2) Unlike the HECT domain ligase that serves as a direct intermediate between E2 and substrate ubiquitin transfer, the RING type functions as a molecular scaffold that brings proteins together instead of acting as a chemical catalyst. It is defined by a conserved series of histidine and cysteine residues that is patterned to form a cross-brace structure, which permits the binding of two zinc cations.