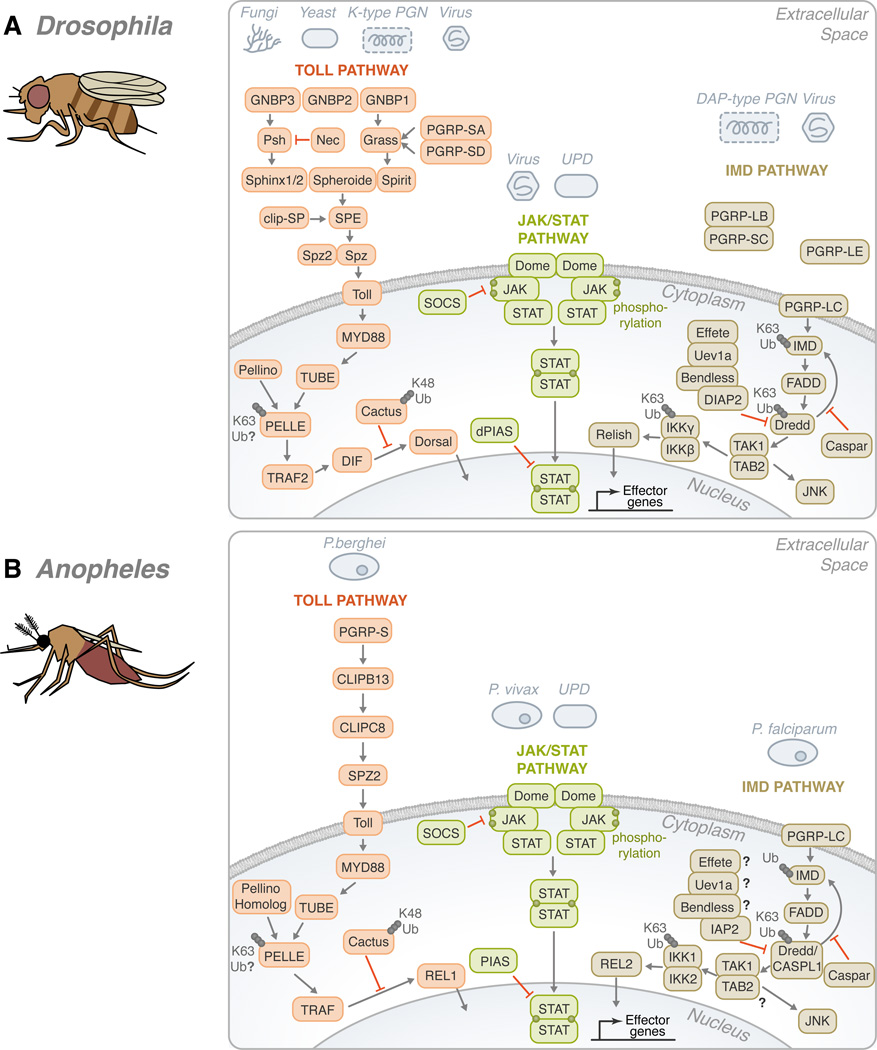
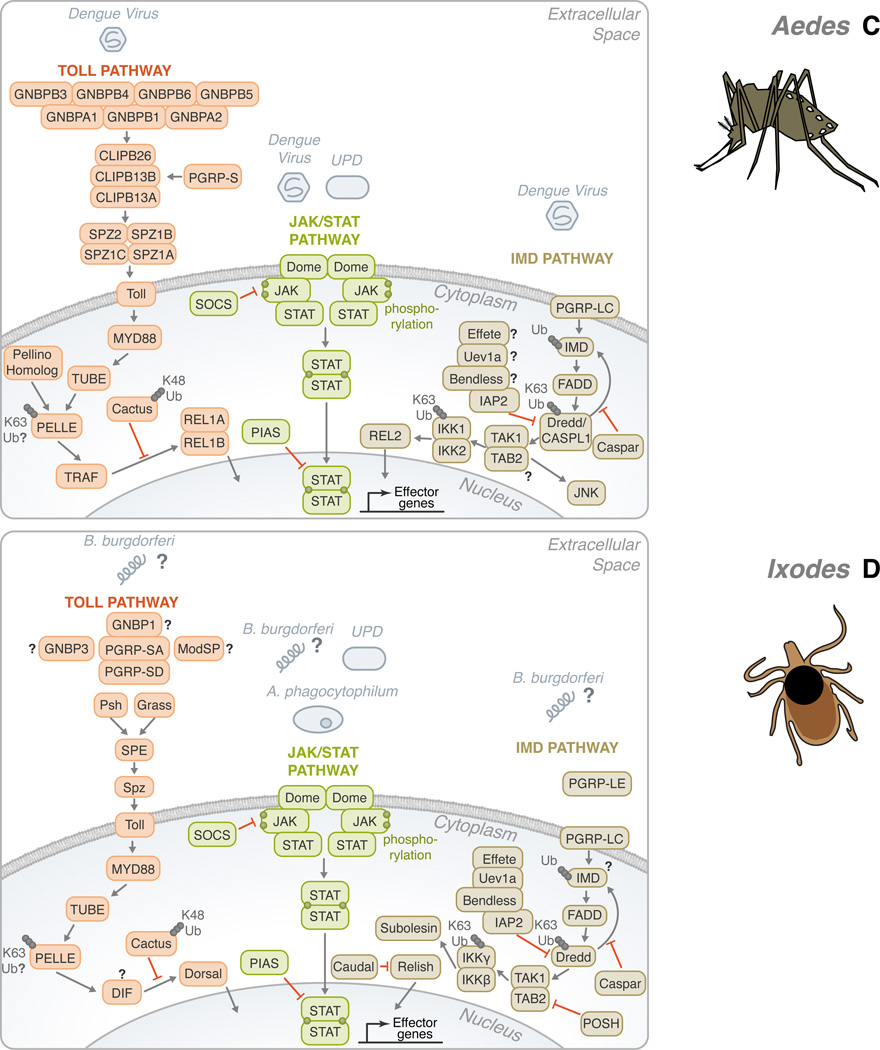
Figure 2. Conservation of the Toll, IMD, and JAK/STAT pathways in Drosophila melanogaster, Anopheles gambiae, Aedes aegypti and Ixodes scapularis.
(A) In Drosophila, gram-negative bacteria binding proteins (GNBPs) and peptidoglycan recognition proteins (PGRPs) have been shown to activate the Toll pathway in the presence of stimulants, such as fungi, yeast, lysine-type peptidoglycan (K-type PGN) and viruses. These recognition proteins signal downstream to Persephone (psh) and Grass. CLIP domain serine proteases (clip-SP) modulate the signaling after recognition as well. Sphinx1/2, Spheroide, and Spirit initiate the activation of Spätzle through the Spätzle processing enzyme (SPE). Spätzle binds to Toll which recruits three Death domain-containing molecules: MyD88, Tube, and Pelle. Pellino/Pellino homolog, perhaps, acts as a positive regulator of immunity by ubiquitinating Pelle. After which, TNF-receptor-associated factor (TRAF) signals to Dorsal-immune related factor (DIF), followed by signaling to Dorsal. The activation is facilitated by the degradation of Cactus through K48 ubiquitination. The transcription factor translocates to the nucleus in order to upregulate immune genes. The Toll pathway is highly conserved in: (B) Anopheles, (C) Aedes, and (D) Ixodes. The Toll pathway has been demonstrated to recognize Plasmodium berghei in (B) Anopheles and the Dengue virus in (C) Aedes. TRAF signaling initiates REL1 and REL1A /1B activity in (B) Anopheles and (C) Aedes, respectively. (A) On the other hand, the Drosophila IMD pathway recognizes primarily mono-diaminopimelic acid-type peptidoglycans (DAP-type PGN). Fas-associated protein with death domain (FADD) is recruited to IMD. FADD binds to the Death related ced-3/Nedd2-like caspase (Dredd)/CASPL1. Dredd can cleave IMD. Inhibitor of apoptosis (IAP) can also associate with Dredd/CASPL1. Effete, Uev1a, and Bendless play a role in the regulation of this step and caspar may also inhibit the activity of IMD-dependent transcription factors. TGF-β activated kinase (TAK1), TAK1 binding protein 2 (TAB2) complex forms as signaling continues. Two avenues may result from the IMD pathway: JNK or NF-κB. For NF-κB activation, Relish translocates to the nucleus to activate effector genes. There are several potential sites of ubiquitination throughout the IMD pathway: IMD, Dredd/CASPL1, and the IKK complex. Like the Toll pathway, the IMD pathway is found in many species: (B) Anopheles, (C) Aedes, and (D) Ixodes. P. falciparum and the dengue virus can trigger the IMD pathway in (B) Anopheles and (C) Aedes, respectively. While Relish is regulated by the IMD pathway in (A) Drosophila and (D) Ixodes, REL2, the homolog of Relish, acts as the transcription factor in (B) Anopheles and (C) Aedes. The third pathway is the JAK/STAT pathway. (A) A ligand derived from the unpaired (UPD) gene activates the pathway by binding to Domeless (Dome). Phosphorylated JAK promotes the dimerization of STAT. Dimerized STAT can proceed to the nucleus. Countering the activation, both SOCS and PIAS negatively regulate the JAK/STAT pathway. Though the JAK/STAT pathway is evolutionarily conserved across the organisms discussed, various pathogens have demonstrated the ability to activate the JAK-STAT pathway, such as: (B) Plasmodium vivax, (C) dengue virus and (D) A. phagocytophilum. For the Toll, IMD and JAK/STAT pathways, B. burgdorferi recognition in I. scapularis remains mostly elusive. The information illustrated here was obtained from gene sequences available at vectorbase (www.vectorbase.org) and the following articles: (Waterhouse et al., 2007, Liu et al., 2012, Souza-Neto et al., 2009, Cirimotich et al., 2010, Xi et al., 2008, Hoffmann, 2003).