Figure 1.
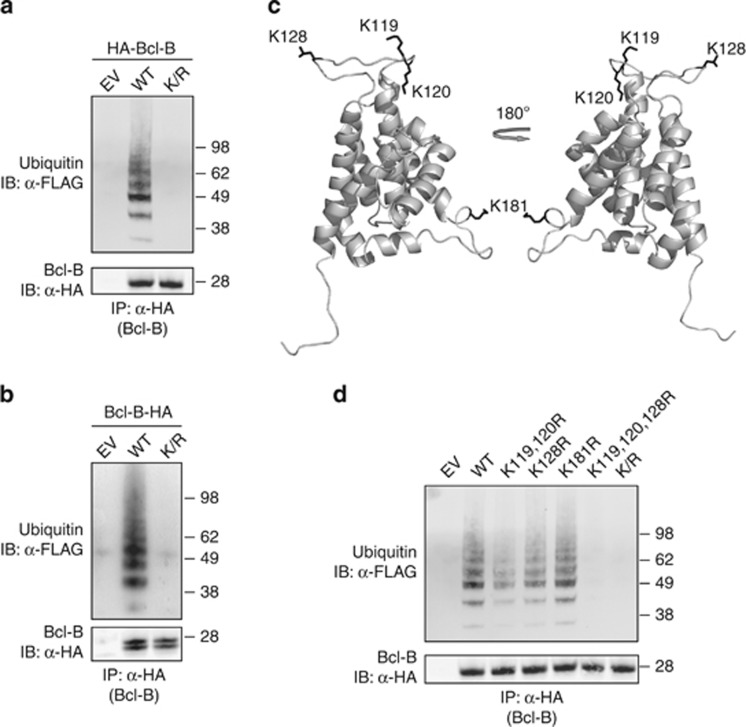
Bcl-B is ubiquitinated on lysine residues. (a) HEK 293 T cells were transfected to express WT or lysine mutant (K/R) N-terminally HA-tagged Bcl-B, or empty vector, together with FLAG-tagged ubiquitin. Bcl-B was isolated from denatured cell lysates by anti-HA IP, followed by immunoblotting of the precipitates. HA-tagged Bcl-B and FLAG-ubiquitin were detected by probing with, respectively, anti-HA and anti-FLAG antibodies. (b) As in a, now with expression of C-terminally HA-tagged Bcl-B in WT and lysineless K/R mutant form. The two Bcl-B proteins species detected in this case result from alternative use of ATG start codons.10 (c) Bcl-B protein structure was modeled using M4T software. Depicted is a cartoon representation of the model structure, with the N terminus facing downwards. Lysine residues with side chain are represented in black. (d) As in a, now with expression of N-terminally HA-tagged Bcl-B in the form of WT, lysineless K/R mutant and various indicated single-, double- or triple K/R point mutants. Data shown are representative of at least three independent experiments.