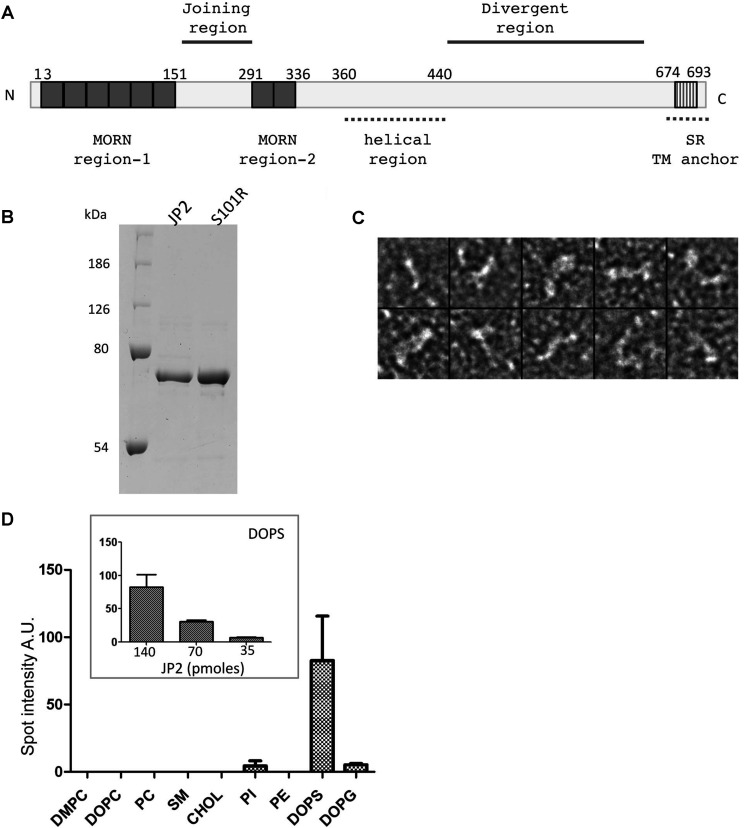
Figure 1. Purification of JP2 and analysis of lipid-binding characteristics.
(A) Putative domains of JP2 based on the primary sequence. (B) Coomassie-Blue-stained SDS/PAGE (10% gel) of purified wild-type JP2 and mutant S101R showing a single polypeptide band at ~75 kDa. Molecular masses are indicated in kDa. (C) TEM images of negatively stained JP2 showing that the protein forms filament structures ~15 nm in length and 2 nm in diameter; box size=25 × 25 nm. (D) Densitometric analysis of lipid–protein overlay (n=3). The inset illustrates that the binding of JP2 to DOPS is quantitative. A.U., absorbance units; CHOL, cholesterol; PI, PtdIns; SR, sarcoplasmic reticulum; TM, transmembrane.