Abstract
Introduction
Type 2 diabetes mellitus (T2DM) progression often results in treatment intensification with injectable therapy to maintain glycemic control. Using pilot data from the Initiation of New Injectable Treatment Introduced after Anti-diabetic Therapy with Oral-only Regimens study, real-world treatment patterns among T2DM patients initiating injectable therapy with insulin glargine or liraglutide were assessed.
Methods
This was a retrospective analysis of claims from the OptumInsight™ (OI; January 1, 2010 to July 30, 2010) and HealthCore® (HC; January 1, 2010 to June 1, 2010) health insurance databases. Baseline characteristics, health care resource utilization, and costs were compared between adults with T2DM initiating injectable therapy with insulin glargine pen versus liraglutide. Follow-up outcomes, including glycated hemoglobin A1c (A1C), hypoglycemia, health care utilization, and costs, were assessed.
Results
At baseline, almost one in three liraglutide patients (OI, n = 363; HC, n = 521) had A1C <7.0%, while insulin glargine patients (OI, n = 498; HC, n = 1,188) had poorer health status, higher A1C (insulin glargine: 9.8% and 9.1% versus liraglutide: 7.9% and 7.7%, OI and HC, respectively, both P < 0.001), and were less likely to be obese (insulin glargine: 10.8% and 9.2% versus liraglutide: 17.4% and 18.8%, OI and HC, respectively, both P < 0.01). The percentage of patients experiencing a hypoglycemic event was numerically higher for insulin pen use for both cohorts (OI 4.4% versus 3.0%; HC 6.2% versus 2.3%). During follow-up, in the insulin glargine cohort, annualized diabetes-related costs remained unchanged ($8,344 versus $7,749 OI, and $7,094 versus $7,731 HC), despite a significant increase in pharmacy costs, due to non-significant decreases in medical costs, while the liraglutide cohort had a significant increase in annualized diabetes-related costs ($4,510 versus $7,731 OI, and $4,136 versus $7,111 HC; both P < 0.001) due to a non-significant increase in medical costs coupled with a significant increase in pharmacy costs.
Conclusion
These descriptive data identified differences in demographic and baseline clinical characteristics among patients initiating injectable therapies. The different health care utilization and cost patterns warrant further cost-effectiveness analysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-013-0074-8) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes mellitus, Health care costs, Injectable treatment, Treatment initiation
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease. Although treatment with an oral anti-diabetic drug (OAD), usually metformin, in combination with lifestyle changes is recommended as the initial therapy for most patients, worsening β-cell function typically requires intensification of therapy with basal or intermediate-acting insulin (e.g., insulin glargine, insulin detemir, or neutral protamine Hagedorn) or glucagon-like peptide-1 (GLP-1) receptor agonists (such as liraglutide) [1]. Initiating injectable therapy may be delayed by negative perceptions from patients and health care professionals, including fear of injection pain, misconceptions about insulin risks (e.g., hypoglycemia and weight gain), attributing the need for insulin to personal failure, and the perceived negative impact on the patient’s social life and job [2–4].
Insulin has traditionally been administered using a vial and syringe. Delivering insulin via pen devices may help address some of the barriers to insulin initiation, including improved convenience and discretion and ease of use [5, 6] that may ultimately also improve outcomes [7–9]. The GLP-1 receptor agonist, liraglutide, is dispensed only in a prefilled pen device, and the insulin analog, insulin glargine, is also available in a prefilled pen device.
The Initiation of New Injectable Treatment Introduced after Anti-diabetic Therapy with Oral-only Regimens (INITIATOR) study expands on earlier clinical trial data [10] by investigating real-world outcomes among patients with T2DM failing OADs and initiating injectable therapy with insulin glargine or liraglutide, both delivered by prefilled pen device. This analysis reports treatment pattern data from the pilot retrospective study phase of INITIATOR.
Methods
Study Design and Patients
This retrospective analysis used medical and pharmacy claims, enrollment information, and linked electronic laboratory results from two independent administrative claims databases associated with OptumInsight™ (OI; Eden Prairie, MN, USA) and HealthCore® (HC; Wilmington, DE, USA) in the United States (US). Commercially insured adults (and Medicare Advantage adults in the HC database) were eligible for the study if they were aged 18 years or older and had T2DM, defined as having one or more inpatient visits or two or more outpatient visits (≥30 days apart) with a primary or secondary diagnosis of T2DM [International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes: 250.x0 or 250.x2], as used in previously published claims-database studies [11–13]. To be included, patients were required to have initiated either insulin glargine by prefilled pen device or liraglutide (with index date as the earliest fill) between January 1, 2010 and June 30, 2010 (HC) or July 31, 2010 (OI) and have received one or more OAD in the 6 months before the index date (baseline). Patients were required to have continuous medical and pharmacy benefits for the baseline period and 9 (OI) or 12 (HC) months after index date (follow-up). In the OI database only, one or more glycated hemoglobin A1c (A1C) values in the baseline period through 15 days following the index date was also an inclusion criterion. In the A1C analyses, for consistency, a sub-sample of patients with available baseline A1C values was used in the HC database as well.
Patients were excluded from the study if they met any of the following criteria: one or more pharmacy claims for insulin, exenatide, liraglutide, or pramlintide during the baseline period; pharmacy claims for both insulin glargine (in either pen or vial form) and liraglutide on the index date; pharmacy claims for insulin glargine in prefilled pen format and insulin glargine in other forms (vial-and-syringe, reusable pen) on the index date. Patients with Medicare Supplemental or Part D were excluded, as not all claims are available for these patients; the HC database retained patients with Medicare Advantage enrollment, as they have full claims representation.
This retrospective analysis is based on previously collected data and does not involve any new studies of human subjects performed by any of the authors.
Baseline Measures
Baseline factors were recorded, including demographics, comorbidities [including modified Quan–Charlson comorbidity index (QCI) [14] and obesity as identified with ICD-9-CM codes], hypoglycemic events, prescription drug usage, and, where available, A1C. Health care resource utilization included emergency department (ED) visits, inpatient hospitalizations, ambulatory visits, and endocrinologist visits, as well as any hospitalization in the 30 days before the index date. Diabetes-related health care resource utilization was also analyzed and included claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx). Health care costs were computed as the combined patient- and plan-paid amounts of adjudicated claims. Costs were adjusted to 2011 US dollars using the medical care component of the US consumer price index [15]. Diabetes-related health care costs included costs from medical claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx), anti-diabetic medications, glucose meters, and test strips.
Endpoint Measures
Clinical outcomes analyzed at follow-up (9 months for OI and 12 months for HC) were A1C change from baseline, treatment persistence, daily average consumption (DACON), and hypoglycemia. Treatment persistence was defined as remaining on the study drug during the follow-up period without discontinuation or switching after study drug initiation [16–19] and medication was considered discontinued if the prescription was not refilled within the expected time of medication coverage (the 90th percentile of the time, stratified by the metric quantity supplied, between first and second fills among patients with at least one refill in the specific cohort, irrespective of post index eligibility). Patients who restarted their initial medication after having met the criteria for discontinuing their index medication were considered to be non-persistent. For patients using the insulin glargine pen, persistence rates were based on all insulin glargine fills because patients on insulin glargine pen could switch to vial-and-syringe as their insulin delivery device but would still be on insulin glargine treatment. DACON was calculated as the total amount of medication (units or mg) dispensed before the last refill of the study drug divided by the total number of days between initiation and last refill during follow-up. While DACON is not comparable between insulin glargine and liraglutide medications, it was reported as part of the respective cohort profiles. Hypoglycemia was defined as a health care encounter (outpatient, inpatient, or ED visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycemia (ICD-9 code 250.8x–diabetes with other specified manifestations; 251.0–hypoglycemic coma; 251.1–other specified hypoglycemia; or 251.2–hypoglycemia, unspecified) [20].
Due to the differences in the baseline demographics and clinical characteristics of these treatment groups, clinical outcomes were described, and health care resource utilization and costs were compared between baseline and follow-up within each treatment group, rather than comparing outcomes between treatment groups. Follow-up health care costs (adjusted to 2011 US dollars) and resource utilization were also computed, using definitions consistent with those created in the baseline period.
Statistical Analysis
This study used an intent-to-treat approach, in which patients who augmented or switched from their initial treatment regimen were retained in their assigned cohort. This approach best captures what the prescribing physician intended for the patient to take. Furthermore, any deviations from the initial treatment are reflective of how the medication is being used in the real-world setting. Baseline characteristics, treatment patterns, and outcomes among the insulin glargine pen and liraglutide groups were assessed descriptively. Baseline characteristics and study outcomes were compared using unadjusted statistical comparisons for both study cohorts. Continuous variables were compared using Student t tests (for the OI cohort) or Student t tests and Wilcoxon rank-sum tests (for the HC cohort), while categorical variables were compared using Fisher exact tests (for the OI cohort) and χ 2 tests (for the HC cohort), depending on the distribution of the measure. Due to demographic and clinical differences observed in the insulin glargine pen and liraglutide cohorts during the baseline period, follow-up outcomes were assessed descriptively. Within each treatment group, annualized diabetes-related health care costs were compared between the baseline and follow-up periods using paired t tests. The statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA; 2008).
Results
Baseline Patient Characteristics
Data from a total of 2,570 patients were included: 861 OI (insulin glargine pen n = 498, liraglutide n = 363) and 1,709 HC (insulin glargine pen n = 1,188, liraglutide n = 521). There were substantial differences in the demographics and baseline clinical characteristics between patients initiating insulin glargine pen and those initiating liraglutide (Table 1). In both databases, patients initiating insulin glargine pen were more likely to be male, have poorer baseline health (as measured by the modified QCI), and be treated with sulfonylureas at baseline. Patients initiating treatment with liraglutide were more likely to be obese or have hyperlipidemia.
Table 1.
Baseline characteristics (intent-to-treat population)
| Characteristic | OI database | HC database | ||||
|---|---|---|---|---|---|---|
| Insulin glargine (n = 498) | Liraglutide (n = 363) | P value | Insulin glargine (n = 1,188) | Liraglutide (n = 521) | P value | |
| Age, years, mean (SD) | 53.1 (9.38) | 52.8 (8.33) | 0.598 | 56.9 (11.90) | 53.2 (9.02) | <0.001 |
| Age category, years, n (%) | ||||||
| 18–39 | 44 (8.8) | 25 (6.9) | 80 (6.7) | 39 (7.5) | ||
| 40–64 | 414 (83.1) | 321 (88.4) | 0.097 | 860 (72.4) | 448 (86.0) | <0.001 |
| 65–74 | 37 (7.4) | 17 (4.7) | 148 (12.5) | 32 (6.1) | ||
| ≥75 | 3 (0.6) | 0.0 | 100 (8.4) | 2 (0.38) | ||
| Male, n (%) | 295 (59.2) | 185 (51.0) | 0.018 | 685 (57.7) | 249 (47.8) | <0.001 |
| QCI, mean (SD) | 0.99 (1.64) | 0.61 (1.13) | <0.001 | 1.05 (1.67) | 0.73 (1.28) | <0.001 |
| Comorbidities, n (%) | ||||||
| Hypoglycemia* | 16 (3.2) | 6 (1.7) | 0.191 | 39 (3.3) | 12 (2.3) | 0.273 |
| Obesity | 54 (10.8) | 63 (17.4) | 0.007 | 109 (9.2) | 98 (18.8) | <0.001 |
| Hypertension | 334 (67.1) | 260 (71.6) | 0.157 | 749 (63.1) | 345 (66.2) | 0.209 |
| Hyperlipidemia | 372 (74.4) | 304 (83.8) | 0.001 | 693 (50.3) | 355 (68.1) | <0.001 |
| Neuropathy | 53 (10.6) | 17 (4.7) | 0.001 | 112 (9.4) | 37 (7.1) | 0.117 |
| Nephropathy | 26 (5.2) | 8 (2.2) | 0.032 | 57 (4.8) | 23 (4.4) | 0.730 |
| Retinopathy | 41 (8.2) | 18 (5.0) | 0.075 | 123 (10.4) | 30 (5.8) | 0.002 |
| Hypoglycemia, events per 100 patient years | 11 | 4 | 16 | 6 | ||
| Glycated hemoglobin A1c, mean, % (SD)a | 9.78 (2.19) | 7.93 (1.56) | <0.001 | 9.09 (1.90) | 7.68 (1.38) | <0.001 |
| OADs, n (%) | ||||||
| Metformin | 407 (81.7) | 304 (83.75) | 0.467 | 889 (74.8) | 441 (84.6) | <0.001 |
| DPP-4 inhibitor | 158 (31.7) | 129 (35.5) | 0.243 | 354 (29.8) | 136 (26.1) | 0.120 |
| Meglitinide derivative | 22 (4.4) | 8 (2.2) | 0.092 | 60 (5.1) | 13 (2.5) | 0.016 |
| Sulfonylurea | 322 (64.7) | 157 (43.3) | <0.001 | 749 (63.1) | 223 (42.8) | <0.001 |
| Thiazolidinedione | 174 (34.9) | 148 (40.8) | 0.087 | 390 (32.8) | 187 (35.9) | 0.218 |
| α-Glucosidase inhibitor | 4 (0.8) | 3 (0.8) | 1.000 | 15 (1.3) | 4 (0.8) | 0.369 |
| Number of OADs, mean (SD) | 2.18 (0.87) | 2.06 (0.92) | 0.053 | 2.07 (0.89) | 1.93 (0.85) | 0.003 |
| Diabetes-related health care utilization, n (%) | ||||||
| Emergency department visit | 28 (5.6) | 3 (0.8) | <0.001 | 71 (6.0) | 25 (4.8) | <0.001 |
| Endocrinologist visit | 122 (24.5) | 103 (28.4) | 0.209 | 252 (21.2) | 168 (32.3) | 0.324 |
| Ambulatory visit | 470 (94.4) | 347 (95.6) | 0.531 | 1,106 (93.1) | 499 (95.8) | 0.033 |
| Hospitalization <30 days before initiation | 27 (5.4) | 1 (0.3) | <0.001 | 45 (3.8) | 4 (0.77) | <0.001 |
OI: insulin glargine n = 498, liraglutide n = 363; HC: insulin glargine n = 283, liraglutide n = 113
OAD oral anti-diabetic drug, SD standard deviation, QCI modified Quan–Charlson Comorbidity Index
* Patients with ≥1 hypoglycemic event. aAmong patients with A1C test results available
Baseline A1C measures were available for all patients included from the OI database, and in this database the insulin glargine pen cohort had a higher mean A1C than the liraglutide group (insulin glargine: 9.8% and 9.1% versus liraglutide: 7.9% and 7.7%, OI and HC, respectively, both P < 0.001; Table 1). Prior to injectable therapy initiation, A1C was already within the American Diabetes Association target range of <7.0% [1] for more of the liraglutide-using patients than the insulin glargine pen initiators (29.5% versus 9.2%, P < 0.001), while baseline A1C was ≥9.0% for fewer of the liraglutide initiators compared to insulin glargine pen initiators (21.8% versus 60.0%, P < 0.001; Fig. 1). Among patients in the HC database with baseline A1C data available [insulin glargine pen n = 283 (24%), liraglutide n = 113 (22%)], baseline mean A1C levels were also higher among those using the insulin glargine pen compared to liraglutide. In this subset, baseline A1C was also <7.0% for more patients using liraglutide than insulin glargine pen (33.6% versus 11.3%, P < 0.001) and ≥9.0% for more patients with insulin glargine pen than liraglutide (47.7% versus 16.8%, P < 0.001). Hypoglycemia during the baseline period was infrequent in both groups (Table 1).
Fig. 1.
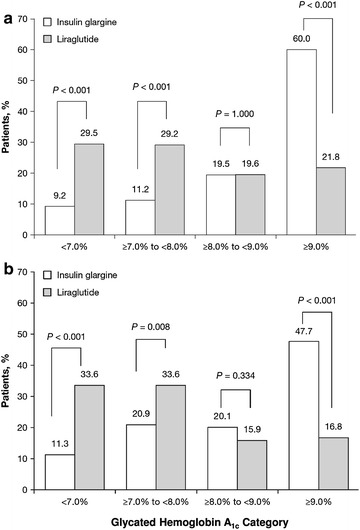
Glycated hemoglobin A1c category at baseline for patients initiating insulin glargine or liraglutide in the OptumInsight (a) and HealthCore (b) databases
Baseline health care utilization and costs were significantly different between cohorts, with a higher percentage of insulin glargine patients experiencing diabetes-related ED visits and hospitalizations in the 30 days prior to injectable therapy initiation (Table 1). Annualized all-cause total health care costs at baseline were either similar or higher for the insulin glargine pen cohort versus the liraglutide cohort, depending on the database [OI $15,050 (median $5,708) versus $10,812 ($6,541), P = 0.020; HC $15,899 ($6,637) versus $11,912 ($7,608), P = 0.137]. The annualized diabetes-related costs for insulin glargine pen initiators compared to liraglutide initiators followed a similar pattern [OI $8,344 ($2,269) versus $4,510 ($2,503), P = 0.006; HC $7,094 ($2,478) versus $4,136 ($2,164), P = 0.126; Fig. 2].
Fig. 2.
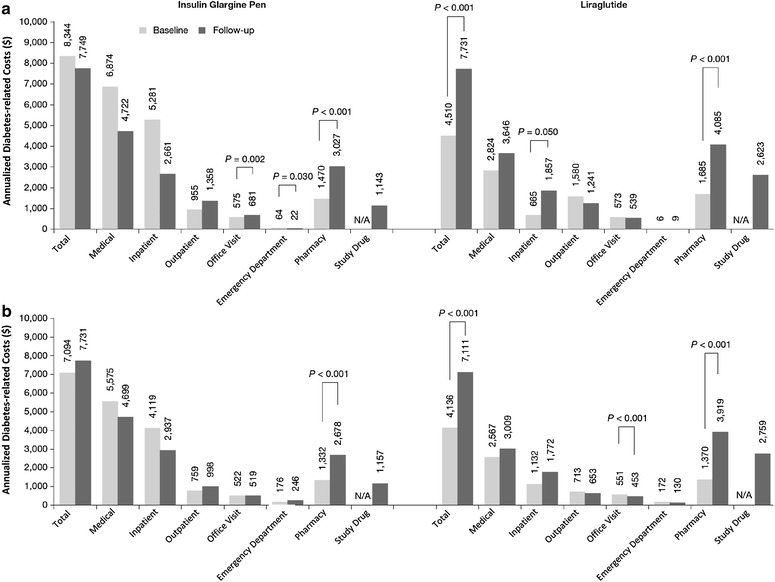
Annualized mean diabetes-related health care costs at baseline and during follow-up among insulin glargine and liraglutide patients from the OptumInsight (a) and HealthCore (b) Databases. All other differences were not statistically different. Diabetes-related health care costs included costs from medical claims with a primary or secondary diagnosis of diabetes (ICD-9-CM: 250.xx), or pharmacy claims for diabetes medication including oral anti-diabetes drugs, insulin, glucagon-like peptide-1 receptor agonists, and pramlintide. N/A not applicable
Follow-up Clinical and Economic Outcomes
For those patients with A1C values available at both baseline and follow-up, mean A1C reduction with insulin glargine pen was 1.11% over 9 months (OI; n = 253 with mean baseline A1C = 9.65%) and 0.75% over 12 months (HC; n = 86 with mean baseline A1C = 8.97%). Mean A1C reduction with liraglutide was 0.58% over 9 months (OI; n = 174 with mean baseline A1C = 8.00%) and 0.38% over 12 months (HC; n = 40 with mean baseline A1C = 7.61%). In patients with A1C ≥7.0% at baseline (OI: insulin glargine n = 229 with mean baseline A1C = 9.98%, liraglutide n = 125 with mean baseline A1C = 8.66%; HC: insulin glargine n = 79 with mean baseline A1C = 9.20%, liraglutide n = 26 with mean baseline A1C = 8.31%), A1C <7.0% was reached during follow-up in 21% and 15% with insulin glargine and 34% (OI) and 38% (HC) with liraglutide.
Treatment persistence among patients using insulin glargine was 61% in the OI database and 60% in the HC database. For patients using liraglutide, treatment persistence was 52% in the OI database and 51% in the HC database. Mean persistence duration was 233 days and 305 days for insulin glargine and 207 days and 264 days for liraglutide in the OI (follow-up 270 days) and HC databases (follow-up 365 days), respectively (Fig. 3). The DACON of insulin glargine was 29.9 and 28.0 IU, and the DACON for liraglutide was 1.14 and 1.44 mg, for the OI and HC databases, respectively.
Fig. 3.
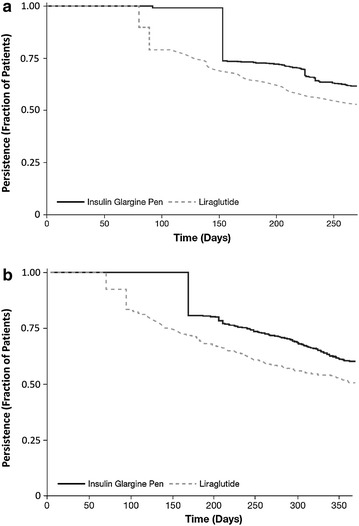
Kaplan–Meier curve for the time to treatment discontinuation for insulin glargine and liraglutide in the OptumInsight (a) and HealthCore (b) databases
The hypoglycemia event rate for insulin glargine pen users was 0.11 (OI database) and 0.25 (HC database) events per patient year; for liraglutide users, it was 0.06 events per patient year in both databases. The percentage of patients experiencing a hypoglycemic event in the 9-month follow-up of the OI database was 4.4% and 3.0% for the insulin glargine pen and liraglutide cohorts, respectively. For the HC database the percentage experiencing at least one hypoglycemic event in the 12-month follow-up was 6.2% with insulin glargine pen and 2.3% with liraglutide.
During 9-month (OI database) and 12-month (HC database) follow-up, respectively, a diabetes-related ED visit occurred in 5% and 12% of patients with insulin glargine and 2% and 7% of patients with liraglutide. Furthermore, a diabetes-related ambulatory visit occurred in 97% and 98% of patients with insulin glargine and 94% and 98% of patients with liraglutide. Finally, an endocrinologist visit occurred for 33% and 30% of patients with insulin glargine and 31% and 34% of patients with liraglutide. Total annualized all-cause health care costs in the follow-up period for the insulin glargine pen cohort were $16,078 (median $7,449) and $16,466 ($9,183) for the OI and HC databases, respectively. Annualized diabetes-related costs among insulin glargine pen-using patients remained similar from baseline to follow-up in both the OI and HC databases ($8,344 versus $7,749 OI, and $7,094 versus $7,731 HC; Fig. 2). There was a significant increase in pharmacy costs in the follow-up period in both databases, but this was offset by non-significant decreases in diabetes-related medical costs (Fig. 2). For the liraglutide initiators, total annualized health care costs in the follow-up period were $15,410 ($9,919) and $14,579 ($10,244) in the OI and HC databases, respectively. Annualized diabetes-related costs among patients initiating liraglutide increased from baseline to follow-up in both the OI and HC databases ($4,510 versus $7,731 OI, and $4,136 versus $7,111 HC; both P < 0.001; Fig. 2). There was a significant increase in pharmacy costs for both databases, with smaller increases for medical costs (which did not meet statistical significance), contributing to the higher diabetes-related costs.
Discussion
This retrospective study of real-world treatment of patients with T2DM failing OADs and initiating injectable therapy with insulin glargine pen or liraglutide showed that insulin glargine and liraglutide pen devices are prescribed to very different types of patients. Patients using insulin glargine had a higher mean QCI, a lower prevalence of obesity, and higher mean A1C than those using liraglutide. In addition, more insulin glargine initiators had A1C ≥9.0%, while a greater proportion of patients initiating liraglutide had an A1C <7.0% at baseline. These baseline differences suggest that a significant number of patients initiating liraglutide may be more likely to have achieved glycemic control prior to the initiation of injectable therapy, and therefore the medication may not have been prescribed primarily for this purpose. When A1C is ≥9.0%, insulin is considered to be more effective than most other agents as a third line therapy [1], which may also account for the high mean A1C levels found in those initiating insulin glargine pen in this study. The higher prevalence of obesity among patients initiating liraglutide in this study may also reflect the perception, due to recent reports of clinical trial results [21, 22], that this agent may be beneficial for weight loss; however, data on weight or body mass index (BMI) were not captured in this pilot analysis to confirm this. Such differences in baseline patient characteristics offer challenges to conducting comparative effectiveness research and in interpreting the results of these studies and, therefore, must be taken into account when such comparative effectiveness studies are designed.
Changes in costs between baseline and follow-up after initiating injectable therapy differed between treatment groups. After initiating insulin glargine pen, the diabetes-related health care costs in the follow-up period remained similar to baseline despite an increase in pharmacy costs. This is explained by the offset resulting from a non-significant decrease in diabetes-related medical costs, driven by lower inpatient costs. For patients initiating liraglutide, the diabetes-related health care costs increased by almost 75% in the follow-up period compared to baseline; this was due to increases in both diabetes-related pharmacy and inpatient costs. Direct comparisons in costs between patients initiating treatment with insulin glargine versus liraglutide would not be appropriate without further adjustments, given the likely confounding influence of baseline patient and clinical differences.
The INITIATOR outcomes study, building on current results, aims to address the discrepancies in the baseline demographics and clinical characteristics by selecting patients who could be considered failing on their OAD therapy, with A1C ≥7.0%, and then further matching the patients on their baseline characteristics to remove the selection bias seen here. This follow-up study will then be able to more accurately compare the outcomes between the cohorts.
One of the strengths of the current study is that is it based on real-world data, using both clinical and economic information from patients with T2DM from two large national US claims databases. Thus, the results reflect actual practice rather than the controlled conditions of clinical trials and may be more applicable to conditions faced by health care providers in the real-world setting. The findings of the current analysis are supported by the consistency in patient characteristics and outcomes between the two, large, independent insurer databases, despite some discrepancies in the two populations at baseline. The HC database had a higher mean age, with a higher percentage of patients aged 75 years or older; this is likely due to the inclusion of Medicare Advantage patients in this database. There is also a disparity in the diagnosis of hyperlipidemia between the databases, although the baseline prevalence of hyperlipidemia is consistently higher among liraglutide patients. Variation in the A1C distribution between the two databases was observed as well, with more patients exhibiting an A1C ≥9.0% in the OI database than in the HC database. As a consequence of the differences in their baseline characteristics, patients from the two databases may be representative of different populations and exhibit different outcomes.
Interpreting results from this study is limited by its retrospective, observational design, as the data may be subject to selection bias and confounding and cannot be used to establish causality of drug effect on observed outcomes. In addition, the analyses were based on data from a managed care population, and they may not be representative of other populations or generalizable to all patients with T2DM. Also, Medicare Advantage patients may be different, both from commercially insured patients and from patients on Medicare Supplemental or Part D only. While pharmaceutical claims provide information on the type and dosage of the prescribed medication, no information was available regarding a patient’s actual daily usage of medication, and, therefore, treatment persistence could only be estimated from pharmacy claims data. The presence of a claim for a filled prescription does not indicate whether the medication was actually used or that it was administered as prescribed. Furthermore, this study was conducted on health care claims data that are potentially subject to coding errors. Health care claims data also pose difficulties in obtaining complete medical histories; for example, these databases did not have information on patients’ weight and BMI.
Conclusion
The interim analysis from this real-world study showed significant baseline differences between T2DM patients initiating liraglutide and insulin glargine, suggesting that these agents are being used to treat different patient groups. Insulin glargine is prescribed for patients with less well controlled diabetes who are in need of larger A1C reduction, whereas liraglutide is given to patients with better glycemic control, with weight loss as an apparent treatment goal for some patients. The substantial differences in demographic and baseline clinical characteristics may confound comparative effectiveness research. The next phase of the INITIATOR study will assess outcomes after accounting for these differences in patient groups. While both types of injectable therapy are associated with increased pharmacy costs, total diabetes-related costs were not affected in glargine users but were increased in liraglutide users, suggesting further cost-effectiveness analysis is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Sanofi US Inc. The authors received writing/editorial support in the preparation of this manuscript provided by Nicola Truss, PhD, of Excerpta Medica, funded by Sanofi US Inc.
This work has been partially presented in poster format at: the 94th Annual Meeting of the Endocrine Society (ENDO 2012), June 23–26, 2012, Houston, Texas, USA; the 35th Annual Meeting of the Society of General Internal Medicine (SGIM 2012), May 9–12, 2012, Orlando, Florida, USA; the 15th Annual European International Society for Pharmacoeconomics and Outcomes Research Congress (ISPOR-EU 2012), November 3–7, 2012, Berlin, Germany.
Ms. S. Thayer is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
S. Thayer is an employee of OptumInsight, under contract with Sanofi US Inc.
E. Buysman is an employee of OptumInsight, under contract with Sanofi US Inc.
L. Brekke is an employee of OptumInsight, under contract with Sanofi US Inc.
W. Crown is an employee of OptumInsight, under contract with Sanofi US Inc.
W. Wei is an employee of Sanofi US Inc.
W. Hu is an employee of Sanofi US Inc.
R. Cuddihy is an employee of Sanofi US Inc.
M. Grabner is an employee of HealthCore, Inc., under contract with Sanofi US Inc.
S. Raparla is an employee of HealthCore, Inc., under contract with Sanofi US Inc.
R. Quimbo is an employee of HealthCore, Inc., under contract with Sanofi US Inc.
M. J. Cziraky is an employee of HealthCore, Inc., under contract with Sanofi US Inc.
Compliance with ethics guidelines
This retrospective analysis is based on previously collected data and does not involve any new studies of human subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Inzucchi SE, Nauck M, Bergenstal RM, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national diabetes attitudes, wishes, and needs (DAWN) study. Diabetes Care. 2005;28:2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733–735. doi: 10.2337/dc09-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveria SA, Menditto LA, Ulcickas Yood M, Koo YH, Wells KE, McCarthy BD. Barriers to the initiation of, and persistence with, insulin therapy. Curr Med Res Opin. 2007;23:3105–3112. doi: 10.1185/030079907X242638. [DOI] [PubMed] [Google Scholar]
- 5.Anderson BJ, Redondo MJ. What can we learn from patient-reported outcomes of insulin pen devices? J Diabetes Sci Technol. 2011;5:1563–1571. doi: 10.1177/193229681100500633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyrot M, Rubin RR. Physician perception and recommendation of insulin pens for patients with type 2 diabetes mellitus. Curr Med Res Opin. 2008;24:2413–2422. doi: 10.1185/03007990802278644. [DOI] [PubMed] [Google Scholar]
- 7.Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12:S101–S108. doi: 10.1089/dia.2009.0180. [DOI] [PubMed] [Google Scholar]
- 8.Lee W, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third party managed care claims data. Clin Ther. 2006;28:1712–1723. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Pawaskar MD, Camacho FT, Anderson RT, Cobden D, Joshi AV, Balkrishnan R. Health care costs and medication adherence associated with initiation of insulin pen therapy in medicaid-enrolled patients with type 2 diabetes: a retrospective database analysis. Clin Ther. 2007;29:1294–1305. doi: 10.1016/j.clinthera.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care. 2012;18:721–726. [PubMed] [Google Scholar]
- 12.Solberg LI, Engebretson KI, Sperl-Hillen JM, Hroscikoski MC, O’Connor PJ. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease, and depression. Am J Med Qual. 2006;21:238–245. doi: 10.1177/1062860606288243. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res Clin Pract. 2010;89:189–195. doi: 10.1016/j.diabres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Chained Consumer Price Index for all urban consumers (C-CPI-U) 1999-2008, Medical Care. Series ID: SUUR0000SAM. Washington, DC: U.S. Dept. of Labor, Bureau of Labor Statistics, 2008. http://data.bls.gov/cgi-bin/surveymost?su. Last accessed August 28, 2013.
- 16.Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14:673–680. doi: 10.3111/13696998.2011.605818. [DOI] [PubMed] [Google Scholar]
- 17.Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28:1000–1011. doi: 10.1007/s12325-011-0074-5. [DOI] [PubMed] [Google Scholar]
- 18.Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15:230–236. doi: 10.1089/dia.2012.0253. [DOI] [PubMed] [Google Scholar]
- 19.Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17:845–852. doi: 10.4158/EP10401.OR. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35:1126–1132. doi: 10.2337/dc11-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of < 7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14:77–82. doi: 10.1111/j.1463-1326.2011.01493.x. [DOI] [PubMed] [Google Scholar]
- 22.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


