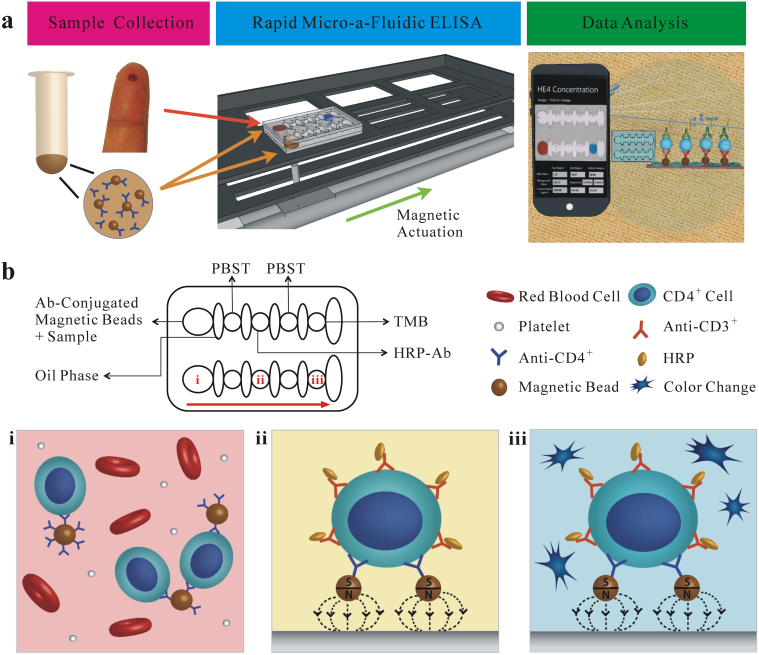
Figure 1. Design of m-ELISA for CD4+ T lymphocyte count.
(a) Overview of the m-ELISA based chip platform. The testing process can be divided into three steps consisting of sample collection, rapid cell-based m-ELISA, and cell phone based colorimetric detection. A droplet from a finger prick can be collected and loaded onto a micro-a-fluidic chip along with antibody-functionalized magnetic beads. The micro-a-fluidic chip is placed on a permanent magnet, which is fixed on a motorized stage. With the aid of a software program, the stage is used to control and complete the entire process of ELISA in an automated manner. As a result, the color development on-chip is imaged using a cell phone and the result is analyzed using an integrated mobile application. (b) Design of a micro-a-fluidic chip and cell-based CD4 ELISA. The chip is designed to contain five aqueous circular chambers (i, ii, iii and two wash chambers containing phosphate buffered saline plus 0.5% tween 20 (PBST)) and five elliptical chambers containing mineral oil for physical separation of the hydrophilic circular chamber liquids. In chamber i, functionalized magnetic beads conjugated with anti-CD4 antibody are used to selectively capture cells (CD4+ T lymphocytes and monocytes) expressing CD4 molecules on the surface. After a washing step in the intermediate chamber, the magnetic beads with captured CD4+ cells are moved to chamber ii and tagged with anti-CD3 antibody, which is conjugated with horseradish peroxidase (HRP). After another wash, the captured CD4+ T lymphocytes enable color development due to digestion of 3,3′,5,5′-Tetramethylbenzidine (TMB) by HRP in chamber iii. The photograph in (a) was taken by ShuQi Wang.