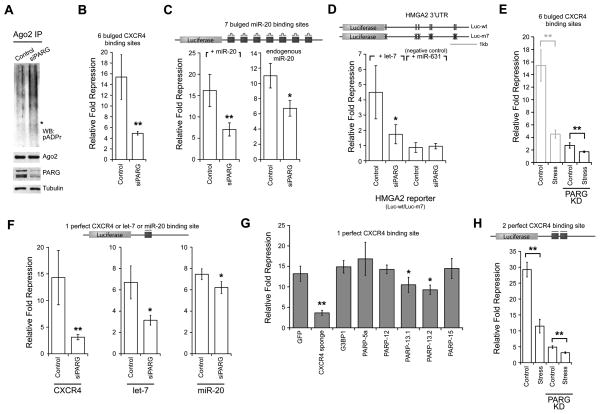
Figure 5. PARG knockdown alleviates miRNA-mediated repression and miRNA-directed cleavage.
(A) pADPr modification levels of endogenous Ago2 in HeLa S3 cells transfected with 25 nM control siRNA or siPARG for 48 hr. Asterisk indicates where Ago2 migrated. Shown are western blots for Ago2, PARG and tubulin. (B) 293T cells were transfected with 25 nM control siRNA or siPARG for 72 hr. Relative fold repression was measured as in Figure 3A; n = 3. (C) PARG knockdown effect observed in luciferase reporter with 7 artificial miR-20 binding sites. The relative fold repression was calculated by the amount of expression of the construct normalized to a construct with all binding sites mutated at their seed positions. The assay was tested with exogenous addition of miR-20 (left) or with endogenous miR-20 (right); n = 4. (D) PARG knockdown effect observed in luciferase reporter with endogenous HMGA2 3′UTR. The relative fold repression is calculated by the amount of expression by the wild-type construct (Luc-wt) normalized to the mutant construct Luc-m7; n = 4. (E) siPARG-transfected cells were either treated with or without 30 nM pateamine A for 2 hr (right). As a comparison, part of Figure 3A is reproduced here on the left to show cells transfected with a control siRNA. (F) The effect of PARG knockdown on miRNA-directed cleavage was examined for luciferase construct with 1 perfect siCXCR4, let-7 or miR-20 binding site; n = 4 in each case. (G) The effect of SG-PARP overexpression on miRNA-directed cleavage assay as in panel F; n = 5. (H) The effect of stress on miRNA-directed cleavage was tested with a luciferase reporter with 2 perfect binding sites for siCXCR4 using the same transfection conditions and drug treatment as in Panel E; n = 3. For panels B-H, error bars indicate SD; paired t-test p < 0.05 (*) and < 0.01 (**). See also Figure S5.