Figure 7.
In trans Docking of a Primer Terminus in the Polymerase Active Site
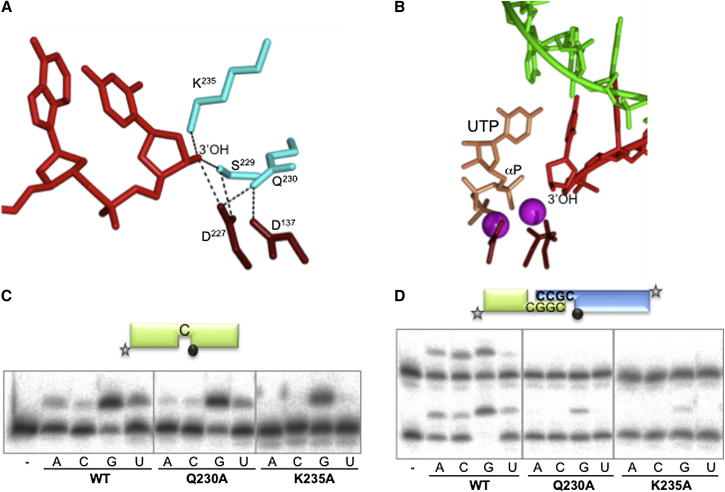
(A) Interaction and stabilization of the 3′-hydroxyl (3′-OH) of the incoming primer within the active site of PolDom. Residues Lys235, Ser229, and Gln230 (cyan) form a network that interacts with the 3′-OH terminus of the primer strand (red). Two of the catalytic aspartates Asp137 and Asp227 (brown) are also part of this network.
(B) A UTP molecule (tan) and catalytic metal ions (magenta), from a PolDom preternary structure (PDB: 3PKY), were superposed into the active site of the annealed break DNA-PolDom complex. The 3′-OH terminus of the primer strand (red) is within nucleophilic attacking distance of the α-phosphate of UTP, providing compelling evidence that this represents a preternary in trans configuration awaiting the arrival of metal ions an incoming base. The templating DNA strand is depicted (green), and the catalytic residues are colored as in (A).
(C) Gap-filling reactions were performed as described in Experimental Procedures for the indicated proteins (25 nM) using a gapped DNA substrate containing the oligonucleotides SP1C, T13C, and DG-P. When indicated, NTPs were added separately at 10 nM in the presence of 1 mM MnCl2.
(D) NHEJ reactions were performed with 600 nM PolDom using a set of DNA substrates formed with the oligonucleotides D3 and NHEJ-D (green, fast running species on the gel) or D4 and NHEJ-D2 (blue, slow running species). Both oligonucleotides were labeled so that primer extension can be observed on both sides of the break at the same time. As indicated, each of the four NTPs (100 μM) was added in the presence of 1 mM MnCl2.
See also Figure S7.