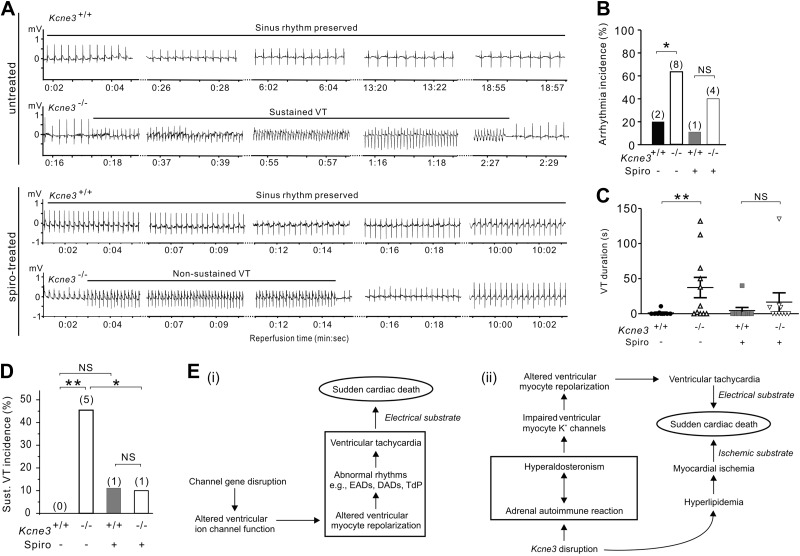
Figure 6.
Kcne3 deletion causes an aldosterone-dependent predisposition to postischemia reperfusion VT. A) Representative (of n=9–10 mice) ECG traces recorded during reperfusion in untreated or spironolactone (spiro)-pretreated 9-mo-old Kcne3+/+ and Kcne3−/− mice. B) Arrhythmia incidence during postischemia reperfusion in 9-mo-old mice with (n=9–10) or without (n=10–11) spironolactone pretreatment. Values in parentheses indicate number of mice exhibiting arrhythmia per group. N.S., not significant (P=0.3 between genotypes). *P < 0.05 between genotypes. C) VT duration (individual indicated by symbols; mean indicated by horizontal line) for mice as in B (mice not exhibiting VT included and indicated as 0 duration). **P < 0.01 between genotypes. D) Sustained (>30 s) VT incidence during postischemia reperfusion in 9-mo-old mice with (n=9–10) or without (n=10–11) spironolactone pretreatment. Values in parentheses indicate number of mice exhibiting sustained VT per group. N.S. = P > 0.05 between genotypes. *P < 0.05. **P < 0.01 between genotypes. E) i) Existing model for the mechanism of monogenic channelopathic predisposition to SCD. ii) Multifactorial etiology of Kcne3-associated arrhythmogenesis and SCD suggested for the Kcne3−/− mouse model, supported by the findings herein. DADs, delayed afterdepolarizations; EADs, early afterdepolarizations; TdP, torsades de pointe.