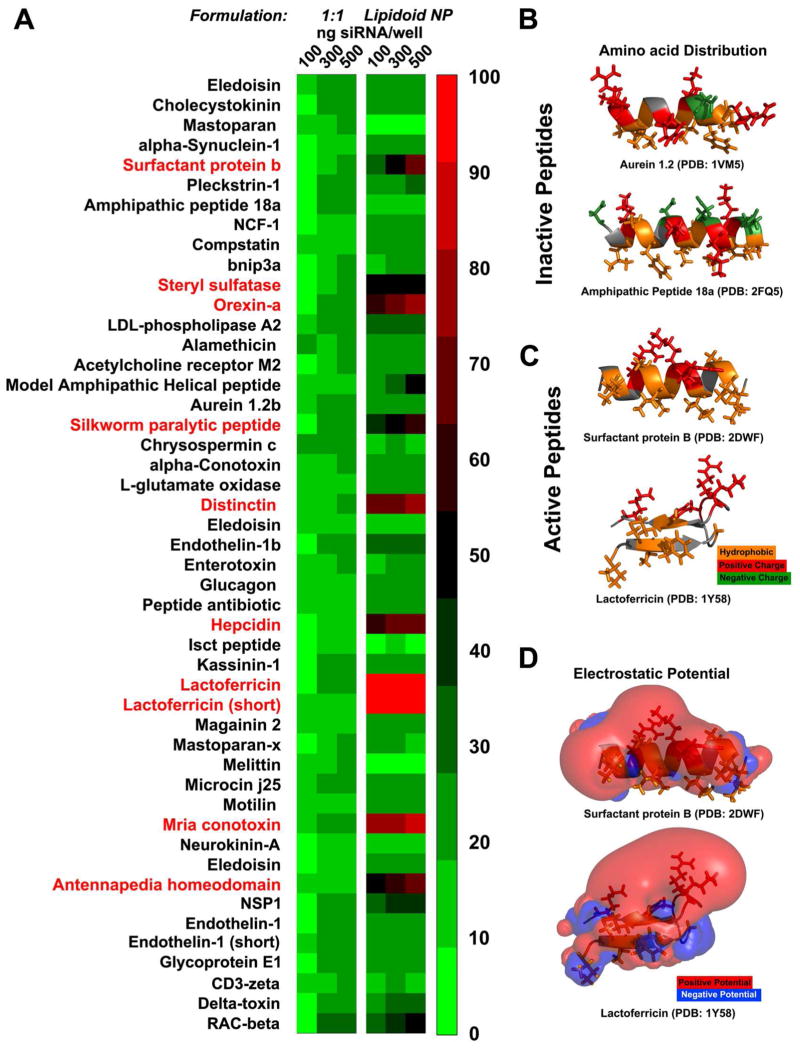
Figure 1. In vitro.
studies of the novel cell penetrating peptides. (A) The 1:1 peptide:siRNA conjugates (left panel) exhibit lower efficacy than the peptide decorated nanoparticles (right panel) at knocking down luciferase in dual HeLa cells. Ten of the peptides (red) exhibited efficacy greater than 60% reduction in the luciferase expression (n=5). (B, C and D) Structure-function correlations of the peptides’ activity. The electrostatic and hydrophobic properties of two inactive and two active peptides can correlate with their activity. The electrostatic potential is visualized as isosurfaces at +1kT/e for positive potential (red) and −1kT/e for negative (blue). Hydrophobic amino acids are represented with orange color while the positively charged with red and the negatively charged with green. In the case of the inactive peptides negatively charged amino acids disrupt the sequence of positively charged residues. For example the peptides derived from aurein 1.2 47, and amphipathic peptide 18a 48 (top and bottom). In the case of active peptides the positively charged amino acids cluster thus creating a surface of positive electrostatic potential that covers almost half of the volume around the peptide allowing the hydrophobic residues to reside in a neutral surface. For example the peptides derived from surfactant protein B 49 (top) and lactoferricin 50 (bottom) are positively charged and only the former is amphiphilic.