Fig. 3.
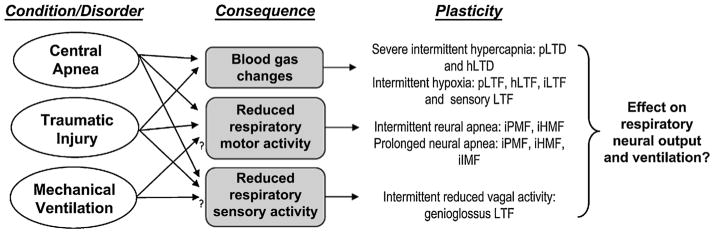
Reduced respiratory neural activity has the potential to elicit multiple forms of plasticity. In non-ventilated animals, central apnea results in reduced respiratory neural activity, profound changes in arterial blood gases and diminished sensory feedback. Similar effects may be observed following disruption of descending inputs to spinal motor neurons (depending on extent and location of disruption). Mechanical ventilation may be associated with reduced respiratory neural activity and/or altered sensory feedback in some patients. Animal models suggest that each of these stimuli independently elicit unique and possibly overlapping forms of plasticity. For example, acute intermittent hypoxia elicits long-term facilitation (LTF) in phrenic, hypoglossal and intercostal nerves (pLTF, hLTF and iLTF, respectively; Bach and Mitchell, 1996; Fregosi and Mitchell, 1994). Additional forms of plasticity are elicited during chronic exposures to intermittent hypoxia, specifically facilitation of carotid body afferent feedback (sensory LTF; Peng et al., 2003). Severe hypercapnia elicits long-term depression of phrenic and hypoglossal nerve activity (pLTD and hLTD; Bach and Mitchell, 1998; Baker et al., 2001). Reduced respiratory neural (motor) activity elicits long-lasting iPMF, and transient iHMF and iIMF (Baertsch and Baker-Herman, 2013; Baker-Herman and Strey, 2011; Mahamed et al., 2011), whereas reduced intermittent sensory (vagal) feedback elicits genioglossus facilitation (Tadjalli et al., 2010). To date, we lack a clear understanding regarding how these multiple forms of plasticity interact to shape necessary long-term adaptations in the respiratory control system.
