Figure 1.
Two PrimPol-like Proteins in Trypanosomatids
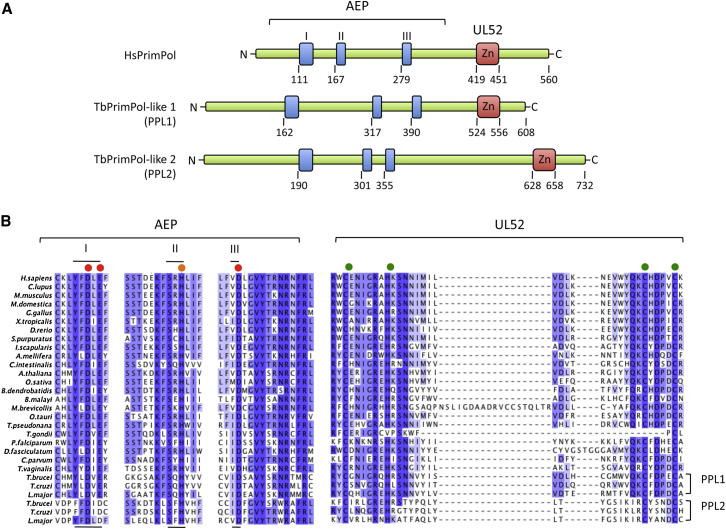
(A) Schematic showing the domain organization of human and T. brucei PrimPols. The highly conserved motifs I, II, and III, comprising the catalytic AEP domain, and the zinc finger with homology to the herpesviral UL52 primase are shown.
(B) Multiple sequence alignments of PrimPol-like proteins from a broad range of multicellular and unicellular eukaryotes. Essential residues in the AEP domain are indicated, residues in motifs I and III (red circle) required for magnesium ion binding, motif II (orange circle) for nucleotide binding. The zinc finger motif is also indicated (green circles). See also Figures S1 and S2.