Figure 4. ULD-ubiquitin interactions.
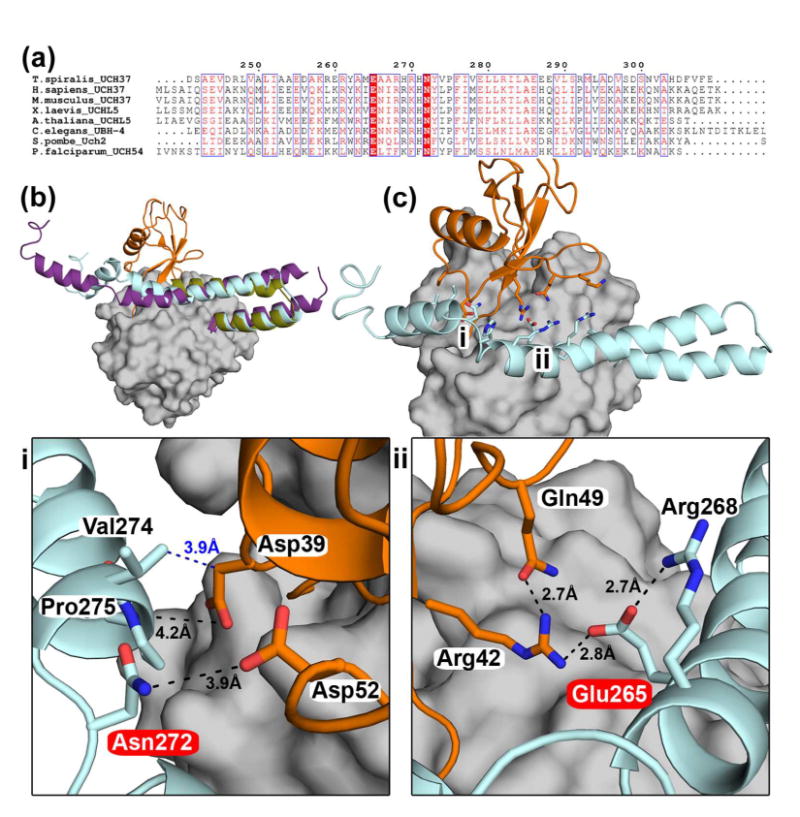
(a) Sequence alignment of the ULD of UCH37 highlighting conserved residues in UCH37 homologs. Glu265 and Asn272 (according to Ts numbering) are absolutely conserved, highlighted in red. (b) Superposition of TsUCH37ΔC46 -UbVME (the ULD in olive, UbVME in orange), human UCH37 (the ULD in purple, PDB ID 3IHR), and TsUCH37 with the entire ULD modeled (cyan) based on the structure of the ULD in human UCH37. The model was generated using SwissModel and MD simulation (please see Materials and Methods). This model is taken from a snapshot collected at 1.3 ns during an MD simulation run of 2 ns. (c) The structure of TsUCH37-ubiquitin complex with the entire ULD modeled as shown in (b), showing that the conserved residues of the ULD could make additional contacts with ubiquitin. The regions marked i and ii are expanded in the panels below. The UCH domain is surface-rendered in grey.
