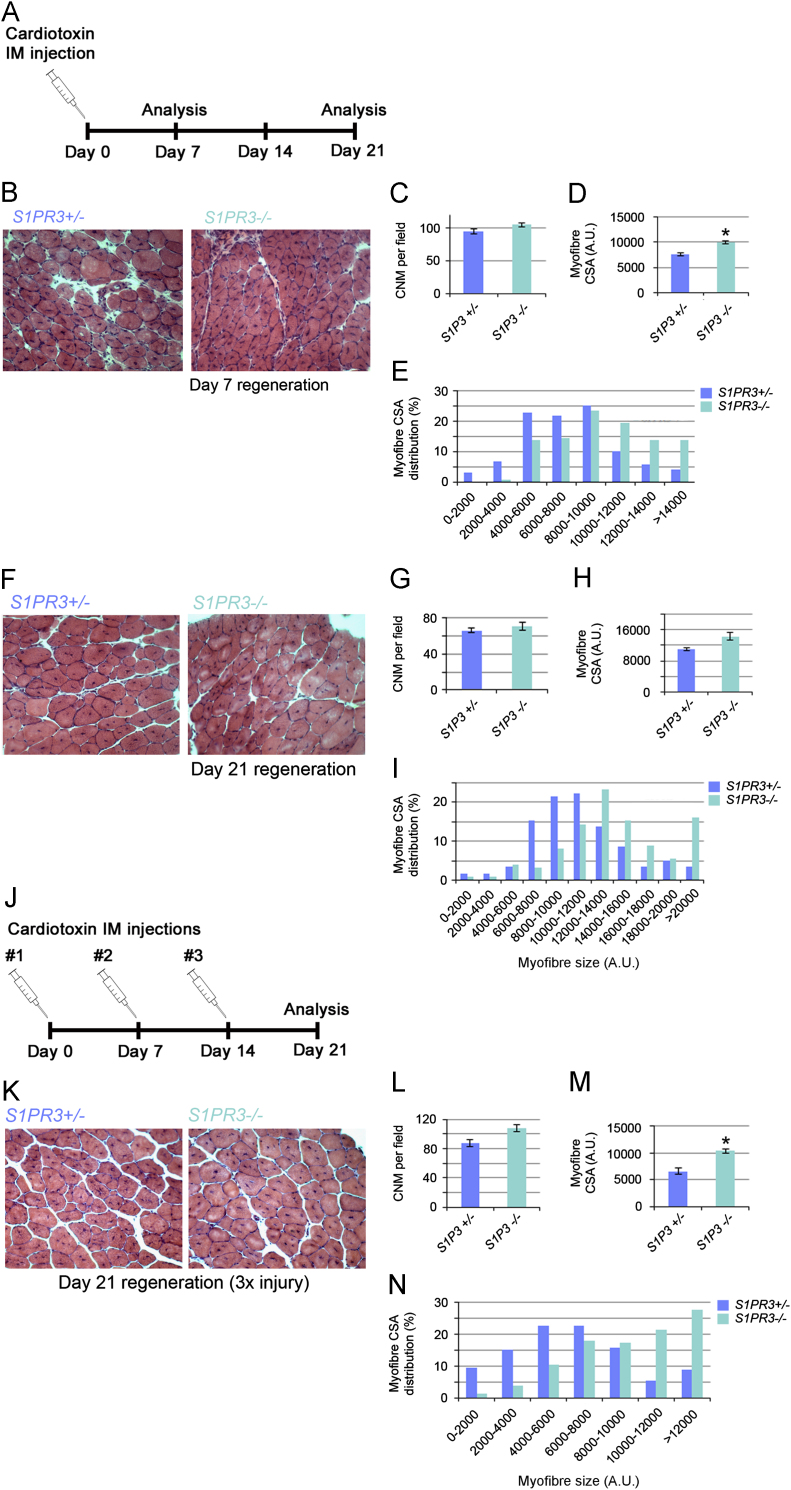
Fig. 4.
Absence of S1PR3 enhances acute muscle regeneration. (A) Both Tibialis Anterior muscles from either age-matched S1PR3+/− or S1PR3−/− mice were injected with cardiotoxin, and removed either 7 or 21 days later, cryosectioned and stained with haematoxylin and eosin. (B)–(C) The presence of myofibres with centrally-located myonuclei (CNM) showed that skeletal muscle regenerated successfully in the absence of S1PR3. (D) - (E) Myofibres had a significantly larger mean cross-sectional area (CSA) in regenerating TA of S1PR3-null mice compared to S1PR3+/− controls, with a bias towards larger size. (F)–(I) After 21 days from the time of injury, there was no longer any difference in the mean size of myofibres. (J) Mice were also subjected to a more rigorous acute regeneration regime of three consecutive rounds of cardiotoxin-induced injury 7 days apart. (K)–(L) Haematoxylin and eosin staining 7 days after the third injury revealed many centrally-nucleated myofibres present, showing that S1PR3−/− mice still regenerated effectively after this more challenging injury routine. (M) - (N) Myofibres in regenerated muscle from S1PR3-null mice had a higher mean cross sectional area than in control heterozygotes with a bias towards a larger size. Values are mean±SEM of a minimum of 40 myofibres from each of 9–13 fields from multiple sections per animal for determining the number of myofibres with centronucleation per unit area, or from between 32 and 68 centrally-nucleated myofibres from a single representative field per animal for measuring CSA, each from 3 mice per genotype. For D, H and M, an asterisk denotes where S1PR3−/− are significantly different (p<0.05) from S1PR3+/− controls using a two-tailed T-test.