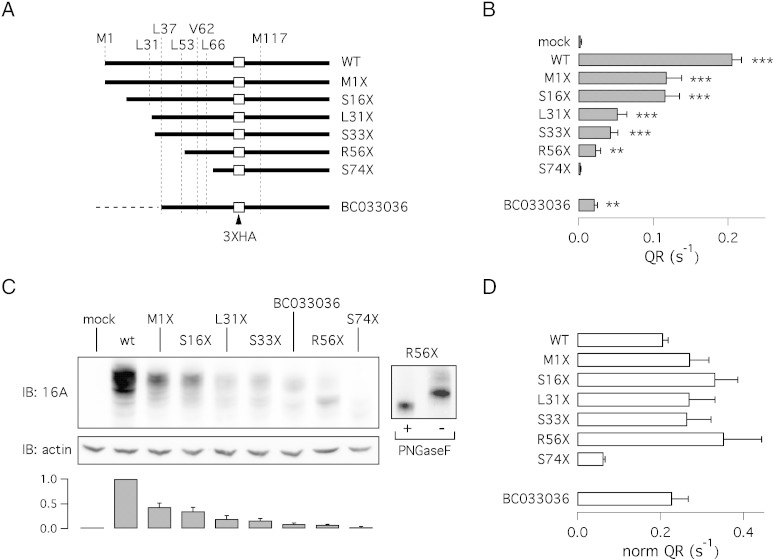
Fig. 3.
Effect of progressive N-terminus truncation. A) Schematic representation of the various truncation mutants used in the study. The position of the two methionines (1 and 117), the HA epitope, and possible (alternative) translation start sites is shown. Additional information about the constructs is also depicted in Fig. 1A. The dashed line reported for the BC033036 construct indicates the presence of the alternative 5′-end which contains three stop codons in frame with the downstream coding sequence. B) Ca2 +-activated halide transport measured for the various constructs using the HS-YFP assay (** and ***, p < 0.01 and p < 0.001, respectively, vs. mock-transfected cells; n = 6). C) Electrophoretic mobility for wild type, BC033036 (TMEM16A(0)), and truncation mutants. All proteins were tagged and detected using the HA epitope. The bar graph shows the densitometric analysis of TMEM16A protein normalized to actin expression (n = 6). For this analysis, we considered the intensity of the band with the highest molecular weight. The inset on the right shows results from an experiment in which lysates from R56X-transfected cells were treated with/without PNGaseF. D) Normalized TMEM16A activity. Anion transport activity (QR) from HS-YFP experiments was normalized to TMEM16A protein expression.