Abstract
Objective:
To evaluate the diagnostic performance of gadoxetic acid-enhanced MRI with an emphasis on the usefulness of the hepatobiliary phase (HBP) in T-staging of gallbladder carcinoma.
Methods:
66 patients with surgically confirmed gallbladder carcinoma underwent MRI. Two radiologists independently reviewed two sets of gadoxetic acid-enhanced MRI without and with the HBP. Local tumour spread was evaluated according to T-staging, and the results were compared with pathological findings. The diagnostic performance of two image sets to differentiate each T-stage was compared.
Results:
The sensitivities of MRI with the HBP to differentiate T1 vs ≥T2 lesions, ≤T2 vs ≥T3 lesions and ≤T3 vs T4 lesions were 96.3%, 85.7% and 100% for Observer 1 and 92.6%, 95.2% and 100% for Observer 2, respectively (p < 0.0001). By adding the HBP, the sensitivities to differentiate ≤T2 vs ≥T3 lesions were increased from 66.7% to 85.7% for Observer 1 and from 81.0% to 95.2% for Observer 2, although there was no significant difference (p > 0.05). The overall accuracies for T-staging were increased from 80.3% to 86.4% for Observer 1, a statistically significant degree (p = 0.046), and from 83.8% to 87.9% for Observer 2 (p > 0.05). The k-value for the two observers indicated excellent agreement.
Conclusion:
Gadoxetic acid-enhanced MRI provided acceptable diagnostic performance for T-staging of gallbladder carcinoma. Addition of the HBP aids in the detection of liver invasion.
Advances in knowledge:
In the T-staging of gallbladder carcinoma, gadoxetic acid-enhanced MRI with the HBP may enhance detection of liver invasion.
Gallbladder carcinoma is the fifth most common gastrointestinal malignancy and the most common biliary tract malignancy worldwide.1 Surgery is the primary treatment of gallbladder carcinoma and remains the only definitive curative therapy, although the curative resection rate ranges only between 10% and 30%.2,3 For early gallbladder carcinoma, some surgeons recommend simple cholecystectomy, whereas others consider radical cholecystectomy to be a curative resection. Advanced gallbladder carcinoma is managed with curative resection, which includes resection of segments IVb and V or even an extended right hepatectomy.4–6 In clinical practice, the optimal type of surgery is determined by the extent of the primary tumour; therefore, T-stage is a critical prognostic factor in gallbladder carcinoma.1 For this reason, it is important to accurately determine the extent of local tumour spread to achieve a successful surgical treatment. CT and MRI and endoscopic ultrasound have been used to determine that extent. Recently, with advances in MR technology and MR contrast media, MRI has become more widely used in the evaluation of hepatobiliary tumours.
Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (gadoxetic acid, Primovist®; Bayer Healthcare, Berlin, Germany) has recently been widely used as an MR contrast agent in the hepatobiliary system and provides dual imaging as an extracellular fluid space contrast agent during the early vascular phase and a liver-specific hepatocellular agent during the delayed phase. With this characteristic feature, gadoxetic acid-enhanced MRI provides homogeneous and strong enhancement of the liver parenchyma in the hepatobiliary phase (HBP), resulting in good contrast between the liver parenchyma and the tumour.7–10 Therefore, the addition of the HBP might be beneficial in the evaluation of the extent of local tumour spread, particularly highlighting minimal liver invasion, because gallbladder carcinoma frequently invades the liver owing to the lack of a muscularis mucosa and submucosa in the gallbladder wall and its direct venous drainage through the liver parenchyma to the hepatic veins.11 To our knowledge, there have been no published reports regarding the performance of gadoxetic acid-enhanced MRI in the pre-operative evaluation of the local spread of gallbladder carcinomas. Therefore, the purpose of this study was to evaluate the diagnostic performance of gadoxetic acid-enhanced MRI and to determine the usefulness of the HBP in the pre-operative T-staging of gallbladder carcinoma.
METHODS AND MATERIALS
Study population
Our study received institutional review board approval, and the requirement for informed consent was waived. Our institutional database was searched for gadoxetic acid-enhanced MR examinations performed in patients with a diagnosis of gallbladder carcinoma between May 2008 and November 2012. This search identified 158 patients. The inclusion criteria were (a) patients who underwent surgery curatively or palliatively for gallbladder cancer and (b) patients who underwent pre-operative MRI within 4 weeks of the surgery. Of the 158 patients, 92 patients were excluded for the following reasons: 39 had no pre-operative MRI, 50 did not undergo surgery and 3 underwent surgery after more than 6 weeks following MRI. Thus, the remaining 66 patients (33 males and 33 females; age range, 36–82 years; mean age, 62 years) were included in the final study group. The mean interval between the time of MRI and surgery was 11.08 ± 11.29 (SD) days.
MR examinations
All MR images were acquired using a 3.0-T whole-body MR system (Intera Achieva 3.0T; Philips Healthcare, Best, Netherlands) with a 16-channel phased-array coil that was used as the receiver coil. The baseline MRI included a T1 weighted turbo field echo in-phase and an opposed breath-hold multishot T2 weighted sequence and a respiratory-triggered heavily T2 weighted sequence.
For contrast-enhanced images, arterial phase (20–35 s), portal phase (60 s), late phase (3 min) and 20-min HBP images were obtained using a T1 weighted three-dimensional turbo field echo sequence (enhanced T1 high-resolution isotropic volume examination, eTHRIVE; Philips Healthcare). The gadoxetic acid was automatically administered intravenously at a rate of 1 ml s−1 and a dose of 0.025 mmol kg−1 body weight using a power injector, followed by a 20-ml saline flush. The time for arterial phase imaging was determined using the MR fluoroscopic bolus detection technique (Bolus Track; Philips Healthcare). The detailed parameters of the MR sequences used are shown in Table 1.
Table 1.
MRI sequences and parameters
| Sequence | TR (ms)/TE (ms) | Flip angle (degrees) | Section thickness (mm) | Matrix size | Bandwidth (Hz/pixels) | Field of view (cm) | Acquisition time (s) | No. of signals acquired |
|---|---|---|---|---|---|---|---|---|
| T1 weighted 2D dual gradient echo | 3.5/1.15–2.3 | 10 | 6 | 256 × 194 | 1918.6/0.226 | 32–38 | 14.0 | 1 |
| Breath-hold multishot T2 weighted | 1623/70 | 90 | 5 | 324 × 235 | 255.3/1.702 | 32–3 | 55.0 | 1 |
| Respiratory-triggered single-shot heavily T2 weighted | 1156/160 | 90 | 5 | 376 × 270 | 388.9/1.117 | 32–38 | 120.0 | 2 |
| T1 weighted 3D gradient echo | 3.1/1.5 | 10 | 2 | 256 × 256 | 723.4/0.601 | 32–38 | 16.6 | 1 |
Image analysis
All images were evaluated independently by two gastrointestinal radiologists: Observers 1 and 2 (with 12 and 3 years' experience in the interpretation of liver MRI, respectively), who were blinded to the histopathological results but knew that all patients had confirmed gallbladder carcinomas. All images were evaluated using a Picture Archiving and Communication System (Centricity™ 3.0; General Electric Medical Systems, Milwaukee, WI) with an adjustment of the optimal window setting in each case. To assess the additional value of the HBP in the pre-operative T-staging of gallbladder carcinoma, at the first reading session, the observers reviewed the gadoxetic acid-enhanced MRI without the HBP (unenhanced, arterial, portal and 3-min late phase). In the second session, the observers reviewed the MRI with the HBP. There was a 4-week interval between the image reviews to minimize any learning bias. The order of case presentation was independently randomized in each session.
We defined the MR criteria of each T-stage according to the American Joint Committee on Cancer TNM classification and related radiological literature concerning T-staging of gallbladder carcinoma, most of which was initially described in CT imaging:12–16 T1, polypoid lesions without focal thickening of the gallbladder wall or nodular/flat lesions with mucosal enhancement; T2, nodular or sessile lesions associated with focal thickening of the gallbladder wall at what was considered to be attachment sites with the presence of an apparently smooth fat plane separating the adjacent organs, focal wall thickening with outer surface dimpling at the tumour base with an apparently smooth fat plane separating the adjacent organs or diffuse thickening of the gallbladder wall with heterogeneous enhancement or two-layered enhancement (composed of strong, thick inner layer enhancement and weak enhancement of the outer layer); T3, apparent nodularity or peritumoral fat infiltration indicating tumour perforating the serosa (visceral peritoneum) and/or directly invading the liver and/or another adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum or extrahepatic bile ducts; and T4, tumour invading the main portal vein or hepatic artery or invading two or more extrahepatic organs or structures. The liver parenchymal invasion was considered when exophytic tumour growth into the liver with/without an indistinct boundary between the liver and tumour was demonstrated. In addition, ill-defined hypointensity in pericholecystic liver parenchyma on the HBP was also considered as liver invasion. Loss of boundary or interface between the tumour and other adjacent organs was used as a diagnostic criterion indicating tumour involvement.12 The diagnostic criteria for vascular invasion on MRI included focal or eccentric luminal narrowing, luminal irregularity, abrupt cut-off of the vascular branches or >50% perimeter contact with the tumour.17,18 After the second review session, the two observers compared the results of each observer with the histopathological findings of surgical specimens as the reference standards and devised possible explanations for the causes of overstaging and downstaging results in consensus.
Reference standard
The final diagnosis of all tumours was based on histopathological examination of the surgical specimen. Exposure of gallbladder cancer and assessment of adjacent organs including liver and vascular involvement were accomplished by surgeons specializing in hepatobiliary surgery during the operation, and histological assessment of the resection margin was performed by one board-certified pathologist. 63 patients underwent curative resection, and the remaining three underwent palliative surgery owing to invasion of the hepatic artery and main portal vein (n = 2) and extensive lymph node metastasis (n = 1). Curative surgical procedures included laparoscopic cholecystectomy (n = 11), open cholecystectomy (n = 8), radical cholecystectomy (n = 10) and cholecystectomy and partial hepatectomy (n = 34). Palliative surgical procedures included exploratory laparotomy, cholecystectomy and cholecystectomy with hepaticojejunostomy.
Statistical analysis
Statistical analyses were performed using two statistical software programs (SAS® v. 9.3; SAS Institute, Cary, NC; and SPSS® v. 18.0; SPSS Inc., Chicago, IL). The diagnostic performance of gadoxetic acid-enhanced MRI to differentiate each T-stage, i.e. T1 vs ≥T2, ≤T2 vs ≥T3 and ≤T3 vs T4 lesions for each observer and each imaging set, was evaluated using Fisher's exact test. The overall accuracies and the sensitivities, specificities, accuracies and positive and negative predictive values to differentiate each T-stage for each observer and each imaging set were calculated. Values of the two imaging sets were then presented as 98.3% confidence intervals (CIs) and compared using the McNemar test for the sensitivities, specificities and accuracies and, in accordance with a previous report,19 for the positive and negative predictive values. p-value < 0.05 was considered statistically significant. The k statistic using the weighted k coefficient was calculated to assess the interobserver agreement.20 k-value < 0.40 indicated marginal reliability; 0.41–0.75, good reliability; and >0.75, excellent reliability.
RESULTS
On histopathological examination, 12 (18.2%) lesions were staged as pT1 (1 pTis, carcinoma in situ; 5 pT1a, tumour confined to mucosal layer; 6 pT1b, tumour confined to muscular layer; size range, 1.5–4.5 cm; mean, 2.6 cm), 33 (50.0%) as pT2 (tumour confined to perimuscular connective tissue; size range, 1.0–10.0 cm; mean, 3.8 cm), 16 (24.2%) as pT3 (size range, 1.3–10.0 cm; mean, 4.7 cm) and 5 (7.6%) as pT4 (size range, 3.0–5.0 cm; mean, 3.8 cm, except 1 T4 lesion, which was not resected). Among 16 tumours with T3, 11 tumours were proven to have liver invasion on pathology. Among five T4 tumours, two were revealed to invade the proper hepatic artery (n = 1) or right hepatic artery (n = 1) and three invaded both the portal vein and hepatic artery. On MRI, grossly, 31 tumours appeared as polypoid masses, 5 were masses replacing the gallbladder and the remaining 30 were seen as localized or diffuse wall thickening.
Table 2 shows the distribution of 66 gallbladder carcinomas according to T-staging on pathology and each MRI set by Observers 1 and 2, respectively. For Observer 1, the weighted k coefficients for correlation between pathology and MRI without/with the HBP image were 0.766 (95% CI: 0.640–0.893) and 0.843 (95% CI: 0.740–0.945), respectively, indicating excellent reliability. For Observer 2, the weighted k coefficients for correlation between pathology and MRI without/with the HBP image were 0.813 (95% CI: 0.703–0.922) and 0.866 (95% CI: 0.775–0.957), respectively, indicating excellent reliability. Thus, there was a significant difference in weighted k coefficient between MRI with and without the HBP for Observer 1 (p = 0.046), but not for Observer 2 (p = 0.083).
Table 2.
Distribution of 66 gallbladder carcinomas according to T-staging on pathology and MRI
| Pathology | Gadoxetic acid-enhanced imaging without/with the hepatobiliary phase |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2 |
3 |
4 |
||||||
| Observer 1 | Observer 2 | Observer 1 | Observer 2 | Observer 1 | Observer 2 | Observer 1 | Observer 2 | ||
| 1 | 10/10 | 11/11 | 2/2 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 12 |
| 2 | 2/2 | 4/4 | 29/29 | 27/27 | 2/2 | 2/2 | 0/0 | 0/0 | 33 |
| 3 | 0/0 | 0/0 | 7/3 | 4/1 | 9/13 | 12/15 | 0/0 | 0/0 | 16 |
| 4 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 5/5 | 5/5 | 5 |
Data represent the number of tumours assigned by MRI without the hepatobiliary phase/the number of tumours assigned by MRI with the hepatobiliary phase.
The diagnostic performance of each imaging set is shown in Table 3 for each observer. For each set of MR images, both observers differentiated T1 vs ≥T2 lesions, ≤T2 vs ≥T3 lesions and ≤T3 vs T4 lesions with a significant degree (p < 0.0001) (Figures 1 and 2). Regarding differentiation of T1 vs ≥T2 lesions and ≤T3 vs T4 lesions, the diagnostic performance between MRI without and with the HBP was equivalent for the two observers. Regarding differentiation of ≤T2 vs ≥T3 lesions, there was a trend towards improved diagnostic performance in the MRI with the HBP compared with the MRI without the HBP, for both observers, although significant differences were not found (p > 0.05). By adding the HBP, the overall accuracies of the T-staging were increased from 80.3% to 86.4% for Observer 1, a statistically significant difference (p = 0.046), and from 83.8% to 87.9% for Observer 2 (p = 0.083).
Table 3.
Diagnostic performancea and comparison between two sets of imaging
| Diagnostic performance | Comparison | Observer 1 |
Observer 2 |
||||
|---|---|---|---|---|---|---|---|
| HBP− | HBP+ | p-valueb | HBP− | HBP+ | p-valueb | ||
| Sensitivity (%) | T1 vs ≥T2 | 96.30 (0.85–0.99) | 96.30 (0.85–0.99) | – | 92.59 (0.79–0.98) | 92.59 (0.79–0.98) | – |
| Specificity (%) | 83.33 (0.49–0.96) | 83.33 (0.49–0.96) | – | 91.67 (0.45–0.99) | 91.67 (0.45–0.99) | – | |
| PPV (%) | 96.30 (0.85–0.99) | 96.30 (0.85–0.99) | – | 98.04 (0.87–0.99) | 98.04 (0.87–0.99) | – | |
| NPV (%) | 83.33 (0.49–0.96) | 83.33 (0.49–0.96) | – | 73.33 (0.43–0.91) | 73.33 (0.43–0.91) | – | |
| Accuracy (%) | 93.94 (0.83–0.98) | 93.94 (0.83–0.98) | – | 92.42 (0.81–0.97) | 92.42 (0.81–0.97) | – | |
| Sensitivity (%) | ≤T2 vs ≥T3 | 66.67 (0.41–0.85) | 85.71 (0.60–0.96) | 0.1365 | 80.95 (0.55–0.94) | 95.24 (0.72–0.99) | 0.2499 |
| Specificity (%) | 95.56 (0.82–0.99) | 95.56 (0.82–0.99) | – | 95.56 (0.82–0.99) | 95.56 (0.82–0.99) | – | |
| PPV (%) | 87.50 (0.58–0.97) | 90.00 (0.64–0.98) | 0.7446 | 89.47 (0.63–0.98) | 90.91 (0.67–0.98) | 0.8199 | |
| NPV (%) | 86.00 (0.71–0.94) | 93.48 (0.79–0.98) | 0.1672 | 91.49 (0.77–0.97) | 97.73 (0.85–0.99) | 0.2820 | |
| Accuracy (%) | 86.36 (0.73–0.94) | 92.42 (0.81–0.97) | 0.1365 | 90.91 (0.79–0.96) | 95.46 (0.85–0.98) | 0.2499 | |
| Sensitivity (%) | ≤T3 vs T4 | 100.00 (0.47–1.00) | 100.00 (0.47–1.00) | – | 100.00 (0.47–1.00) | 100.00 (0.47–1.00) | – |
| Specificity (%) | 100.00 (0.91–1.00) | 100.00 (0.91–1.00) | – | 100.00 (0.91–1.00) | 100.00 (0.91–1.00) | – | |
| PPV (%) | 100.00 (0.47–1.00) | 100.00 (0.47–1.00) | – | 100.00 (0.47–1.00) | 100.00 (0.47–1.00) | – | |
| NPV (%) | 100.00 (0.91–1.00) | 100.00 (0.91–1.00) | – | 100.00 (0.91–1.00) | 100.00 (0.91–1.00) | – | |
| Accuracy (%) | 100.00 (0.92–1.00) | 100.00 (0.92–1.00) | – | 100.00 (0.92–1.00) | 100.00 (0.92, 1.00) | – | |
HBP+, with the hepatobiliary phase; HBP–, without the hepatobiliary phase; NPV, negative predictive value; PPV, positive predictive value.
Data in parentheses are 98.3% confidence intervals.
All data, p < 0.0001, Fisher's exact test.
Bonferroni's correction.
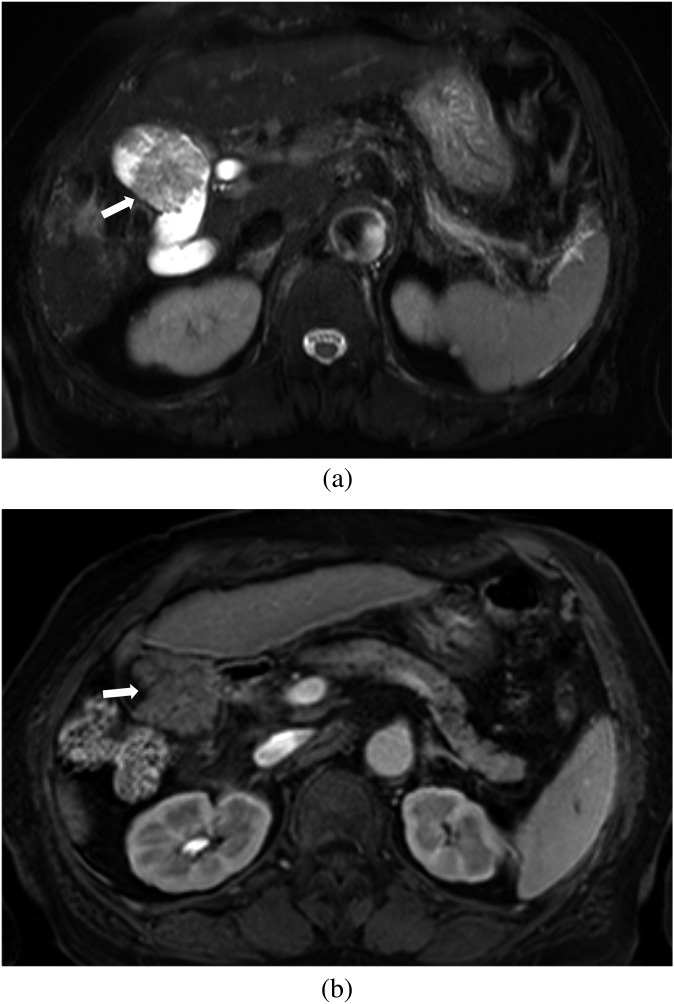
Figure 1.
Images of a 79-year-old female with surgically confirmed T1b gallbladder carcinoma. (a) Axial breath-hold multishot T2 weighted image shows a papillary lesion (arrow). (b) Axial gadoxetic acid-enhanced MR images obtained at the portal phase show an enhancing papillary lesion (arrow). There is no wall thickening around the nodular lesion in the gallbladder. Both observers correctly diagnosed this as a Stage T1 lesion with MRI.
Figure 2.
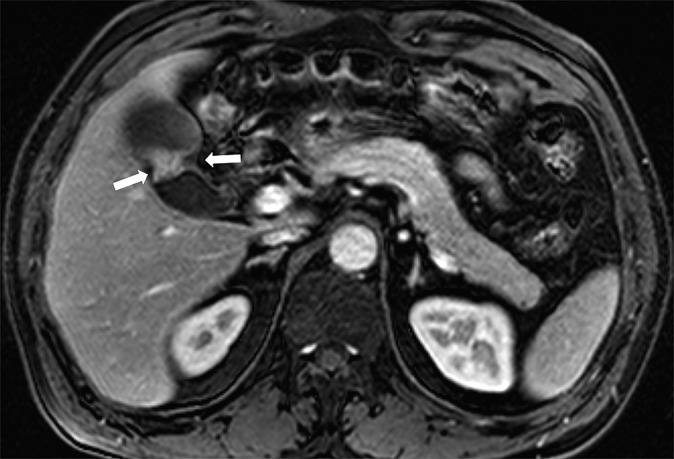
Images of a 57-year-old male with surgically confirmed T2 gallbladder carcinoma. Axial gadoxetic acid-enhanced MR images obtained at the portal phase show well-enhancing focal wall thickening with outer surface dimpling (arrows). There is no peritumoral fat infiltration. Both observers correctly diagnosed this as a Stage T2 lesion on MRI.
Both observers recorded four overstaged lesions in both MRI sets. Among overstaged lesions, two pT1 (one pTis, one pT1b) lesions and two pT2 lesions were misinterpreted as T2 and T3 lesions, respectively. Three tumours (one T1 and two T2) were commonly overstaged in both imaging sets by the two observers. On reviewing these, 2 (50%) pT2 lesions were seen as full-thickness enhancing wall thickening with loss of the fat plane between the tumour and the adjacent organs. On the operative and histopathological findings, we found peritumoral inflammation with adhesion to adjacent structures without evidence of direct tumour invasion. 1 (25%) pTis lesion revealed papillary proliferation and was misinterpreted as a T2 lesion by both observers. The remaining pT1b lesion (25%) was misinterpreted as a T2 lesion by Observer 1 owing to a small vessel along the serosal aspect of the gallbladder wall. There was no case that was correctly discerned by adding the HBP.
Conversely, there were 12 understaged lesions without the HBP. Two pT2 tumours and one pT3 tumour were understaged as T1 and T2, respectively, in both imaging sets by both observers. Among them, 1 (8.3%) pT2 lesion that was misinterpreted as T1 by both observers showed histopathologically microscopic tumour infiltration beyond the muscle layer. A small polypoid tumour with pT2 (8.3%) that was misinterpreted as T1 by both observers accompanied concurrent chronic cholecystitis with multiple gallstones. 2 (16.7%) pT2 lesions showed polypoid lesions with minimal wall thickening at the tumour base. 3 (25.0%) pT3 lesions had subtle tumour infiltration into the serosa. The remaining 5 (41.7%) pT3 lesions showed focal liver invasion in the gallbladder bed of the liver. By the addition of the HBP, four and three pT3 lesions with liver invasion, which were misinterpreted as T2 lesions by Observers 1 and 2, respectively, were correctly discerned as T3 lesions (Figures 3–5).
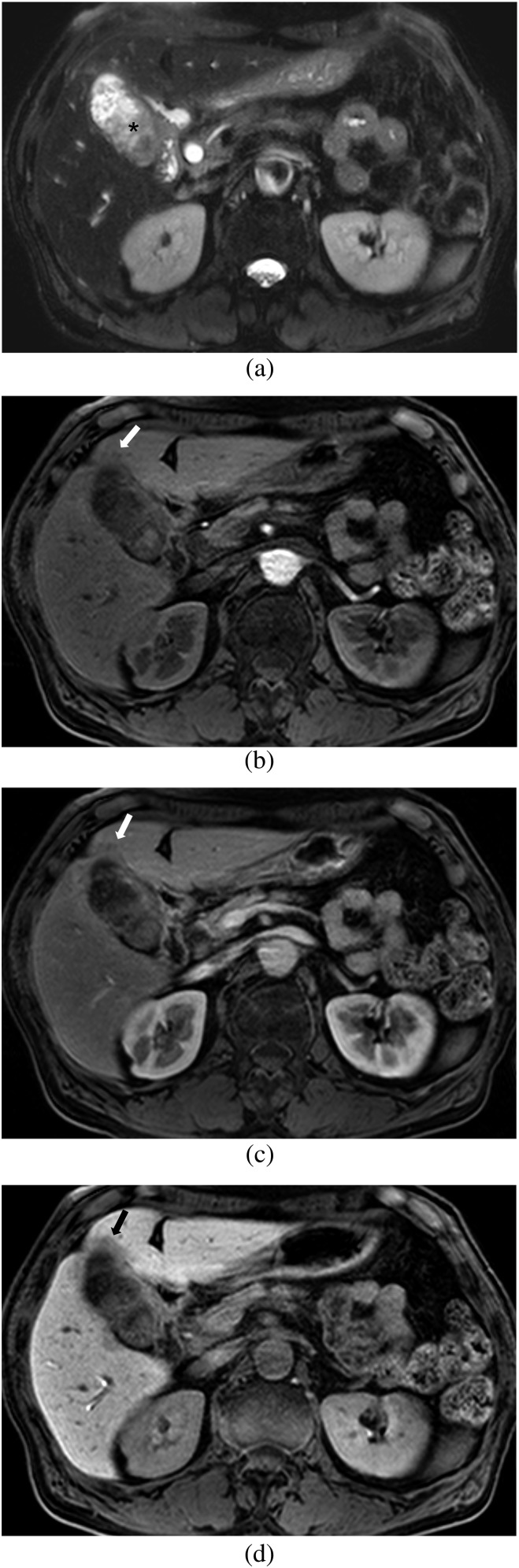
Figure 3.
Images of a 60-year-old male with a surgically confirmed T3 lesion. (a) Axial respiratory-triggered single-shot T2 weighted image shows papillary lesions in the entire gallbladder (asterisk). (b, c) Axial gadoxetic acid-enhanced MR images obtained at (b) arterial phase and (c) portal phase show enhancing papillary lesions with wall thickening of the gallbladder and a subtle low-signal-intensity area (arrow) in segment 4 of the liver. Both observers diagnosed a Stage T2 lesion with no liver invasion on MRI without the hepatobiliary phase (HBP). (d) Axial gadoxetic acid-enhanced 20-min HBP image clearly shows a low-signal-intensity area indicating focal liver invasion of gallbladder cancer into the adjacent liver (arrow). Both observers correctly diagnosed a Stage T3 lesion with focal liver invasion on MRI with the HBP.
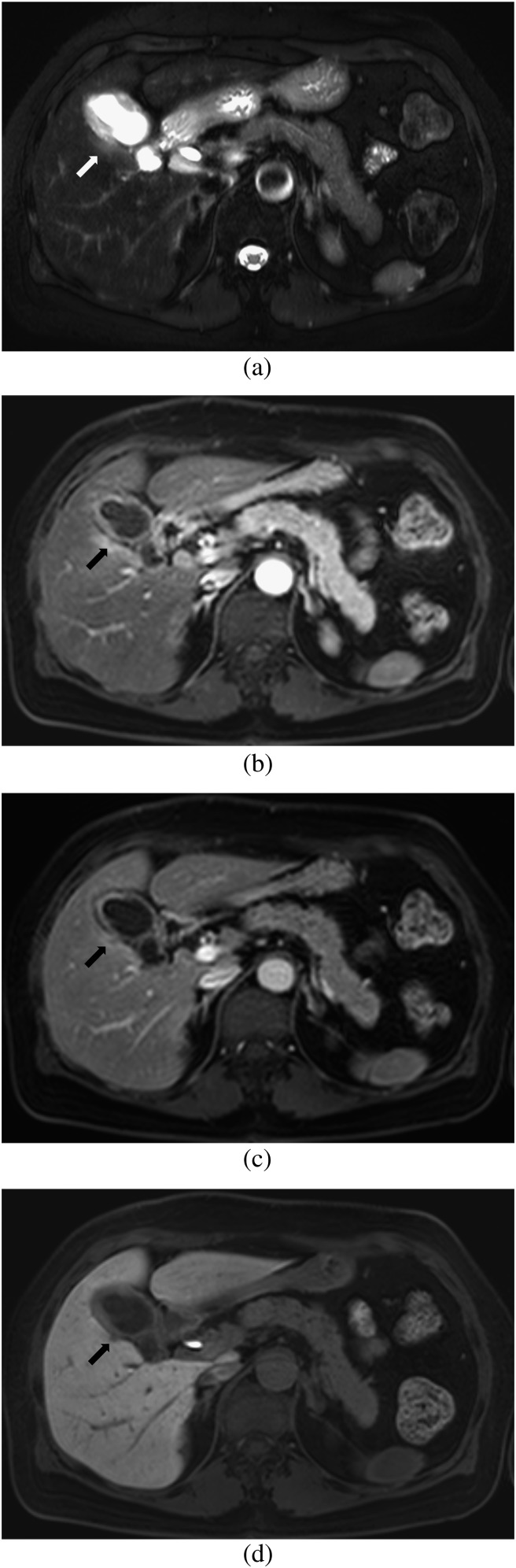
Figure 5.
Images of a 56-year-old male with surgically confirmed T3 gallbladder carcinoma. (a) Axial breath-hold multishot T2 weighted image shows wall thickening of the gallbladder and loss of interface between the tumour and adjacent liver with a high-signal-intensity area in the adjacent liver (arrows). (b, c) Axial gadoxetic acid-enhanced MR images obtained at (b) the arterial phase and (c) the portal phase show loss of interface between the tumour and the adjacent liver and surrounding hyperaemia without obvious exophytic tumour growth in the adjacent liver (arrows). Both observers diagnosed this as a Stage T2 lesion with no liver invasion on MRI without the hepatobiliary phase (HBP). (d) Axial gadoxetic acid-enhanced 20-min HBP image clearly shows a low-signal-intensity lesion indicating focal liver invasion of gallbladder cancer into the adjacent liver (arrows). Both observers correctly diagnosed a Stage T3 lesion with focal liver invasion on MRI with the HBP.
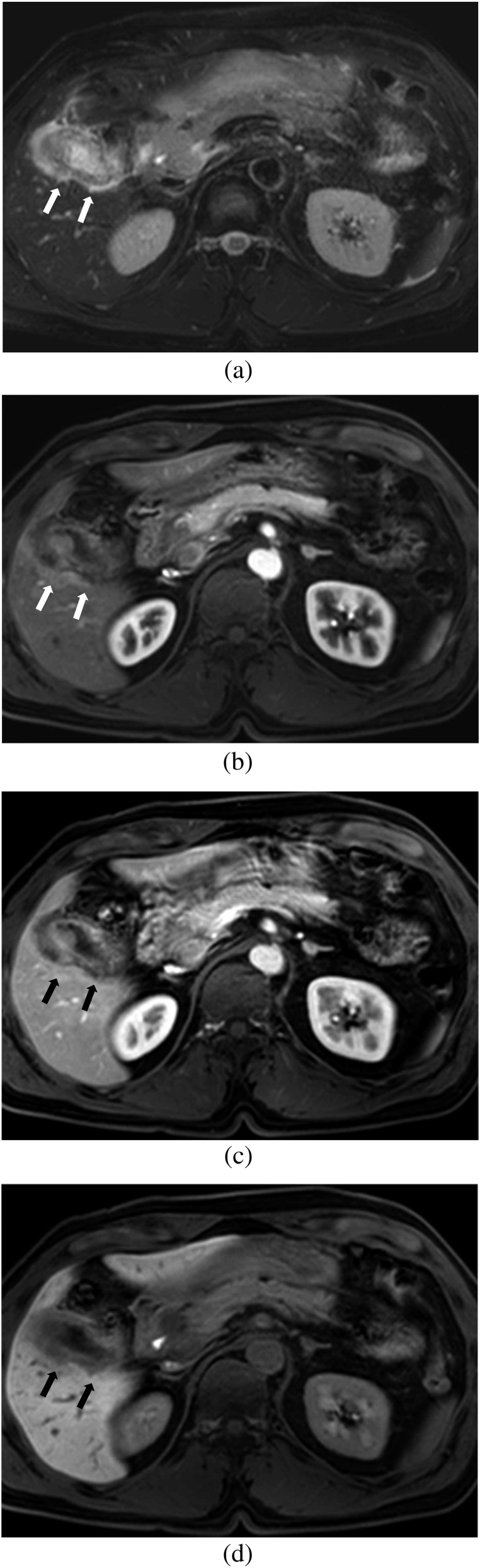
Figure 4.
Images of a 61-year-old female with surgically confirmed T3 gallbladder carcinoma. (a) Axial breath-hold multishot T2 weighted image shows wall thickening of the gallbladder and a high-signal-intensity area in the adjacent liver (arrow). (b, c) Axial gadoxetic acid-enhanced MR images obtained at (b) the arterial phase and (c) the portal phase show a subtle low-signal-intensity lesion (arrow) with surrounding hyperaemia in the adjacent liver. Both observers diagnosed this as a Stage T2 lesion with no liver invasion on MRI without the hepatobiliary phase (HBP). (d) Axial gadoxetic acid-enhanced 20-min HBP image clearly shows a low-signal-intensity lesion indicating focal liver invasion of gallbladder cancer into the adjacent liver (arrow). Both observers correctly diagnosed a Stage T3 lesion with focal liver invasion on MRI with the HBP.
The k-values for the two observers regarding the T-staging of gallbladder carcinoma were 0.859 (95% CI: 0.761–0.956) for MRI without the HBP and 0.916 (95% CI: 0.843–0.989) for MRI with the HBP, indicating excellent agreement.
DISCUSSION
Our study achieved acceptable diagnostic performance with gadoxetic acid-enhanced MRI with the HBP in terms of differentiating T1 vs ≥T2 lesions, ≤T2 vs ≥T3 lesions and ≤T3 vs T4 lesions (p < 0.0001) with the overall accuracies of 86.4% and 87.9% for each observer. This is comparable to the results of a previous study using multidetector CT (MDCT), which showed an overall accuracy of 83.9%.14 Adding the HBP resulted in an improvement in sensitivity in the diagnosis of T3 lesions (66.7 vs 85.7 for Observer 1 and 81.0 vs 95.2 for Observer 2), although significant differences were not found (p > 0.05). Our sample size was relatively small, which limited the power of the data analyses. Statistically significant differences might have been present with a larger sample size. Consequently, by adding the HBP, the overall accuracies were increased from 80.3% to 86.4% for Observer 1, a statistically significant difference (p = 0.046), and from 83.8% to 87.9% for Observer 2 (p = 0.083). This was attributed to the improved detection of focal liver invasion in the gallbladder bed of segments IV or V with the HBP, given 11 of 16 tumours with T3 were revealed to have liver invasion on pathology. Although no tumour overstaged without the HBP was correctly discerned by the addition of the HBP, of the 12 lesions understaged without the HBP, 4 and 3 pT3 lesions with liver invasion misinterpreted as T2 lesions by Observers 1 and 2, respectively, were correctly discerned as T3 lesions by adding the HBP.
Because many investigators in recent studies suggested that aggressive surgery can improve long-term survival, even in patients with advanced stage of gallbladder carcinoma, it has become more important to detect minimal liver invasion.21 A previous study reported that there was a relatively low sensitivity for liver invasion with MRI using conventional gadolinium chelates, whereas there was a high sensitivity for bile duct and vascular invasion of gallbladder carcinoma.17 Gadoxetic acid-enhanced MRI can be taken up by normal hepatocytes at approximately 50% of the injected dose, providing strong enhancement of the liver parenchyma in the HBP image and, in turn, good contrast between the liver parenchyma and the malignant liver tumour, devoid of hepatocyte function.7–10 Thus, HBP imaging can also enhance detection of liver invasion because it provides the highest lesion conspicuity against the strongly enhancing background liver, and is not hampered by accompanying hyperaemia in the gallbladder bed of the liver due to accompanying cholecystitis or aberrant systemic venous drainage, which can obscure or mimic liver invasion. In addition, given that microvessel invasion of a hepatic tumour could cause peritumoral hypointensity on the HBP,22 the pericholecystic liver with tumour invasion on the HBP could be exaggerated by combining hypointensity of tumour invasion as well as peritumoral microvessel invasion. In our cases, five tumours found to have liver invasion with microvessel invasion on pathology demonstrated irregular faint hypointensity in the pericholecystic liver.
Theoretically, gadoxetic acid has a drawback for determining vascular invasion because the dosage of commercially available gadoxetic acid is one-quarter that of conventional gadolinium chelates (0.025 vs 0.1 mmol kg−1). However, five T4 tumours that had invaded the hepatic artery and/or portal vein were correctly discerned by both observers. Although we demonstrated the value of gadoxetic acid-enhanced MRI for the pre-operative T-staging of gallbladder carcinoma, several challenges remain towards lymph node or liver metastasis and peritoneal seeding, which are also important in determining resectability of gallbladder carcinoma.17 Recent studies demonstrated the benefit of gadoxetic acid-enhanced HBP imaging in the detection of small metastases.23 However, when gallbladder carcinoma is accompanied by significant biliary obstruction associated with bile duct invasion, HBP imaging might be limited because the enhancement of the liver parenchyma could be poor. This might be explained by the fact that uptake of gadoxetic acid into the hepatocytes and its excretion into the biliary canaliculi is mediated by the same transporters responsible for intrinsic factors such as bile acid components.9,24 This issue is out of the scope of the current study and should be investigated separately.
Our study had several limitations. First, because our study included only patients who underwent surgery, there might be a selection bias in the patient population towards those with less advanced disease. Second, given that gadoxetic acid is taken up by hepatocytes 60–90 s after administration, gadoxetic acid-enhanced dynamic phases might not fully reflect dynamic imaging using conventional gadolinium chelates.25 Thus, additional efficacy of HBP imaging to conventional dynamic MRI could not be determined in this study. Third, we focused only on local spread of gallbladder carcinomas without considering intra-abdominal distant metastasis. Thus, this study did not show the efficacy of gadoxetic acid-enhanced MRI compared with other imaging modalities such as MDCT in the evaluation of gallbladder carcinoma.
In conclusion, gadoxetic acid-enhanced MRI including the HBP provided acceptable diagnostic performance for the pre-operative T-staging of gallbladder carcinoma. The addition of HBP imaging could be useful in the detection of focal liver invasion.
REFERENCES
- 1.Mekeel KL, Hemming AW. Surgical management of gallbladder carcinoma: a review. J Gastrointest Surg 2007; 11: 1188-93. 10.1007/s11605-007-0115-1 [DOI] [PubMed] [Google Scholar]
- 2.Hamrick RE Jr, Liner FJ, Hastings PR, Cohn I Jr. Primary carcinoma of the gallbladder. Ann Surg 1982; 195: 270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr LH, Wright FH. Carcinoma of the gallbladder. Am Surg 1984; 50: 275-6. [PubMed] [Google Scholar]
- 4.Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery 1987; 101: 731-7. [PubMed] [Google Scholar]
- 5.Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg 2001; 192: 600-7. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor VK, Benjamin IS. Resectional surgery for gallbladder cancer. Br J Surg 1998; 85: 145-6. 10.1046/j.1365-2168.1998.00715.x [DOI] [PubMed] [Google Scholar]
- 7.Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010; 256: 806-16. 10.1148/radiol.10091334 [DOI] [PubMed] [Google Scholar]
- 8.Kim YK, Kim CS, Han YM, Park G. Detection of small hepatocellular carcinoma: can gadoxetic acid-enhanced magnetic resonance imaging replace combining gadopentetate dimeglumine-enhanced and superparamagnetic iron oxide-enhanced magnetic resonance imaging? Invest Radiol 2010; 45: 740-6. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Zech CJ, Bolondi L, Jonas E, Kim MJ, Matsui O, et al. Consensus report of the 4th International Forum for Gadolinium-Ethoxybenzyl-Diethylenetriamine Pentaacetic Acid Magnetic Resonance Imaging. Korean J Radiol 2011; 12: 403-15. 10.3348/kjr.2011.12.4.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park G, Kim YK, Kim CS, Yu HC, Hwang SB. Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinomas: comparison with gadopentetate dimeglumine. Br J Radiol 2010; 83: 1010-16. 10.1259/bjr/66686028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan A, Ferris JV, Hosseinzadeh K, Borhani AA. Gallbladder carcinoma update: multimodality imaging evaluation, staging, and treatment options. AJR Am J Roentgenol 2008; 191: 1440-7. 10.2214/AJR.07.3599 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimitsu K, Honda H, Shinozaki K, Aibe H, Kuroiwa T, Irie H, et al. Helical CT of the local spread of carcinoma of the gallbladder: evaluation according to the TNM system in patients who underwent surgical resection. AJR Am J Roentgenol 2002; 179: 423-8. 10.2214/ajr.179.2.1790423 [DOI] [PubMed] [Google Scholar]
- 13.Kalra N, Suri S, Gupta R, Natarajan SK, Khandelwal N, Wig JD, et al. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol 2006; 186: 758-62. 10.2214/AJR.04.1342 [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Lee JM, Lee JY, Choi JY, Kim SH, Han JK, et al. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am J Roentgenol 2008; 190: 74-80. 10.2214/AJR.07.2348 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimitsu K, Nishihara Y, Okamoto D, Ushijima Y, Nishie A, Yamaguchi K, et al. Magnetic resonance differentiation between T2 and T1 gallbladder carcinoma: significance of subserosal enhancement on the delayed phase dynamic study. Magn Reson Imaging 2012; 30: 854-9. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC cancer staging manual. 7th edn. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 17.Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, et al. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging 2002; 16: 676-84. 10.1002/jmri.10212 [DOI] [PubMed] [Google Scholar]
- 18.Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 2006; 239: 113-21. 10.1148/radiol.2383050419 [DOI] [PubMed] [Google Scholar]
- 19.Bennett BM. On comparisons of sensitivity, specificity and predictive value of a number of diagnostic procedures. Biometrics 1972; 28: 793-800. [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-74. [PubMed] [Google Scholar]
- 21.Dixon E, Vollmer CM Jr, Sahajpal A, Cattral M, Grant D, Doig C, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005; 241: 385-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JW, Daugherty SF. Primary carcinoma of the gallbladder. Surg Clin North Am 1986; 66: 743-9. [DOI] [PubMed] [Google Scholar]
- 23.Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology 2011; 260: 446-53. 10.1148/radiol.11103548 [DOI] [PubMed] [Google Scholar]
- 24.Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 2009; 29: 1707-24. 10.1148/rg.296095501 [DOI] [PubMed] [Google Scholar]
- 25.Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 1996; 200: 59-67. 10.1148/radiology.200.1.8657946 [DOI] [PubMed] [Google Scholar]