Abstract
HIV-specific ADCC antibodies could play a role in providing protective immunity. We have developed a whole blood ADCC assay that measures NK cell activation in response to HIV peptide epitopes. These HIV peptide-specific ADCC responses are associated with escape from immune recognition and slower progression of HIV infection and represent interesting HIV vaccine antigens. However, the mechanism by which these epitopes are expressed and whether or not they induce NK-mediated killing of cells expressing such peptide-antigens is not understood. Herein, we show that fluorescent-tagged ADCC peptide epitopes associate with blood granulocytes. The peptide-associated granulocytes become a specific target for antibody-mediated killing, as shown by enhanced expression of apoptosis marker Annexin and reduction in cell numbers. When HIV Envelope gp140 protein is utilized in the ADCC assay, we detected binding to its ligand, CD4. During the incubation, cells co-expressing gp140 and CD4 reduce in number. We also detected increasing Annexin expression in these cells. These data indicate that blood cells expressing HIV-specific ADCC epitopes are targeted for killing by NK cells in the presence of ADCC antibodies in HIV+ plasma and provide a clearer framework to evaluate these antigens as vaccine candidates.
Keywords: HIV, ADCC, NK cells, granulocytes, apoptosis
Introduction
Developing an HIV vaccine is a global priority. Several lines of evidence suggest antibodies that trigger NK cell mediated killing of virus-exposed cells termed antibody-dependent cellular cytotoxicity (ADCC) could contribute to the prevention or control of HIV infection. Several human cohort studies suggest ADCC antibody responses correlate with slower progression to HIV.1-4 Passive antibody transfer studies in macaques demonstrate a role for ADCC antibodies in controlling SHIV infection.5 Macaque-SIV vaccine studies have suggested a role for ADCC antibodies in protective immunity.6-8 The Thai RV144 human HIV vaccine efficacy trial, which induced high levels of HIV-specific ADCC antibodies, showed partial protection from infection that has been linked to non-neutralizing antibodies.9-11 There is considerable interest in understanding how HIV-specific ADCC could be utilized in an HIV vaccine strategy.9
Most commonly studied in vitro ADCC assays measure the ability of these antibodies to mediate killing of immortalized cell lines expressing HIV proteins.1,7,12 These assays have been important in defining the utility of ADCC antibodies. Our group has described a whole blood based ADCC assay that measures activation of NK cells (e.g., expression of IFNγ or the de-granulation marker CD107) in response to ADCC antibodies in HIV-infected blood and overlapping 15-mer HIV peptides.13,14 Serum transfer experiments showed the activity was mediated by IgG immunoglobulin within the HIV+ serum. Linear HIV ADCC epitopes could be mapped using individual peptides from within the overlapping peptide pool. Using this assay we recently reported the emergence of viral escape variants following ADCC selection pressure15 and that ADCC responses to particular epitopes are associated with slow HIV progression.16 Furthermore, other groups have also reported HIV-specific NK cell activation in reaction to HIV-peptide stimulation.17,18
The mechanism of activation of NK cells by exogenous HIV peptide ADCC epitopes is investigated in this manuscript. In order for ADCC activity to occur, three key components are generally required, namely: (1) target cells that express the HIV antigen, (2) antibodies that bind the viral antigen and (3) effector cells expressing Fcγ receptors, such as NK cells, which bind the Ag-Ab complex. Activated NK cells will secrete a number of cytokines to potentiate the immune response and de-granulate cytolytic molecules to cause apoptosis of the target cell.
The target cells expressing the peptide ADCC epitopes within the whole blood NK cell activation ADCC assay are unclear. Furthermore, it would be advantageous to prove that the cells expressing HIV-peptide antigen are actually killed by NK cells upon activation in the presence of HIV+ plasma. Typical killing-based ADCC assays use immortalized CD4 cell lines exposed to whole viral proteins to measure ADCC activity.10,19 The rapid fluorometric ADCC assay (RFADCC) is based on pulsing a CD4 T cell line with HIV Envelope protein and showing that CD4 cells are target for ADCC related killing. We compared HIV Envelope gp140 protein pulsed CD4 T cells in the RFADCC with Envelope peptide stimulated whole blood in the NK activation ADCC assay. A comparable number of individuals responding to the protein also responded against the peptide antigen.13 Furthermore, comparison of Envelope gp140 protein and Envelope peptides in our NK activation ADCC assay indicated similar percentages of CD107 and IFNγ expression.13 Within the whole blood assay we have described, we envisage that one or more primary blood cells express the peptide epitopes and may serve as a target for NK cell-mediated ADCC in the presence of HIV+ plasma. This study sought to further understand the mechanisms behind HIV-1 peptide specific NK cell activation within the whole blood ADCC assay. We hypothesized that we could identify this population using fluorescently labeled peptide epitopes and this population would specifically undergo apoptosis and reduction in cell numbers in association with NK cell-mediated ADCC in the presence of HIV+ plasma.
Results
ADCC peptide epitope binding is not HLA restricted
We have previously demonstrated that HIV-1 peptide antigens within whole blood samples can induce CD56+ NK cells to liberate cytokines via an ADCC mechanism.14 We have also observed CD107 de-granulation13 however we have not confirmed whether cytotoxicity of target cells is induced. Our previous work has shown that peptide-antibody complexes added to whole blood do not activate substantial numbers of NK cells.13 We hypothesized that a cell population within whole blood express these HIV-1 peptides to ADCC antibodies in HIV+ plasma, which in turn bind and activate NK cells to express IFNγ (Fig. 1A), resulting in killing of the peptide-expressing cell. We have previously shown that cytokine producing cells were not CD3+ T cells, that optimal responses were generated with a peptide length of 14 amino acids or longer and that responses were contingent on the presence of HIV positive IgG and not contingent on the presence of autologous cells.14 Although it seems unlikely that HLA molecules would present peptides for ADCC recognition, we have not previously excluded this possibility.
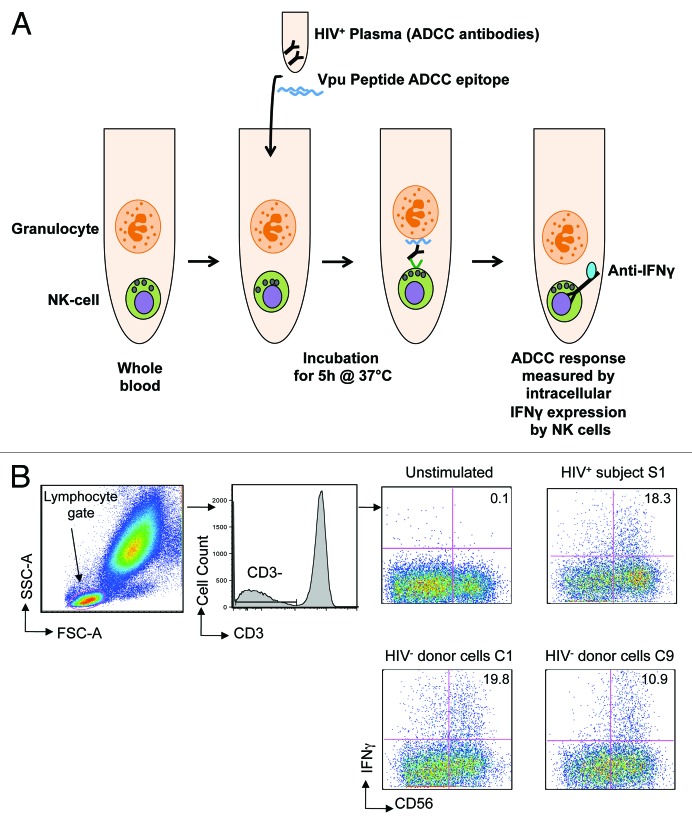
Figure 1. Peptide-based NK cell activation ADCC assay. (A) The assay used whole blood samples in which we theorise that granulocytes expressing HIV peptides activate NK cells in the presence of peptide-specific ADCC antibodies in HIV+ plasma. The ADCC response is measured by intracellular IFNγ expression (previously shown to correlate with CD107 degranulation13) by NK cells in the presence of HIV+ plasma and Vpu peptide ADCC epitope at the end of 5 h incubation at 37°C by flow cytometry. (B) Gating strategy: lymphocytes are identified on forward and side scatter; CD3-CD56+ NK cells are then analyzed for intracellular IFNγ expression. The assay was performed using cells from 9 different HIV negative controls (C1-C9, see Table 1) in the presence of plasma from one HIV+ subject S1; plots are shown for C1 and C9 compared with S1. The value in each plot represents the percentage of CD56+ NK cells expressing IFNγ in the presence of HIV+ plasma following stimulation by Vpu peptide antigen compared with an unstimulated control.
Therefore, we formally examined whether peptide-specific ADCC responses were HLA-restricted. We utilized an HIV-1 subtype B linear ADCC epitope in the HIV-1 Vpu protein (termed Vpu19; sequence EMGHHAPWDVDDL14). This Vpu peptide was incubated with plasma from an HIV positive subject (S1) known to respond to the Vpu19 epitope in the presence of either the subject’s own blood cells or cells from 9 individual HIV negative control subjects (C1-C9). We evaluated NK cell IFNγ expression after a 5-h incubation by gating on CD3-CD56+ lymphocytes (Fig. 1B). The Vpu19 peptide elicited activation of CD56+ NK-cells from HIV+ subject cells (S1; top right plot) and 9-control subjects (C1 and C9 are shown in Fig. 1B, lower plots) in the presence of HIV+ plasma. HLA-A, B, C, DRB1, DQA1, DQB1 typing of S1 and C1-C9 showed that although some alleles were shared by several donors (e.g., A*02:01 by 4 donors), there were no common class I or class II alleles identified among all 9 donors despite the activation of NK cells by ADCC antibodies in all 9 donors (Table 1). These results indicate that presentation of peptides to ADCC antibodies is not dependent on HLA restriction.
Table 1. NK cell activation ADCC response to HIV is not HLA-restricted.
| NK cell donor | HLA A | HLA B | HLA C | HLA DRB1 | HLA DQA1 | HLA DQB1 | ADCC response* |
|---|---|---|---|---|---|---|---|
| S1 | 03:01;26:01 | 27:05;27:05 | 01:02;02:02 | 1,9 | 1,3 | 3,5 | 18.3 |
| C1 | 02:01;68:01 | 51:01;60:01 | 03:04;15:04 | 13,14 | 1,1 | 5,6 | 19.8 |
| C2 | 11:01;24:02 | 48:03;62:01 | 03:03;08:01 | 11,14 | 1,5 | 3,5 | 18.1 |
| C3 | 03:01;33:03 | 44:03;52:01 | 07:01;12:02 | 4,7 | 2,3 | 2,3 | 3.1 |
| C4 | 02:01;03:01 | 35:03;51:01 | 04:01;16:02 | 4,15 | 1,3 | 3,6 | 8.8 |
| C5 | 02:01;23:01 | 44:03;57:01 | 04:01;06:02 | 7,12 | 2,5 | 2,2 | 7.1 |
| C6 | 01:01;02:01 | 08:01;39:01 | 07:01;12:03 | 1,3 | 1,5 | 2,5 | 8.2 |
| C7 | 03:01;29:01 | 07:01;44:01 | 07:01;16:02 | 7,15 | 1,2 | 2,6 | 4.8 |
| C8 | 11:01;24:07 | 38:02;75:21 | 04:03;07:02 | 15,15 | 1,2 | 5,6 | 15 |
| C9 | 29:01;30:02 | 18:01;38:01 | 05:01;12:03 | 3,14 | 1,5 | 2,5 | 10.9 |
*% NK cells expressing IFNγ
Peptides bind to granulocytes inducing killing during the ADCC assay
Identifying cells within whole blood that bind ADCC peptide epitopes and potentially act as peptide-expressing cells was accomplished by using flow cytometry and tracking Vpu19 peptide conjugated with FITC fluorochrome (Vpu-FITC; GL Biochem, Shanghai, China), SIV Gag peptide KC10 (sequence KKFGAEVVPC) was conjugated to AlexaFluor 488 fluorochrome (GL Biochem) and used as a control. The fluorescently labeled Vpu peptide (Vpu19-FITC) was still able to elicit antibody-mediated NK cell-IFNγ expression (Fig. 2A, upper plots). Vpu-FITC bound almost exclusively to cells with high forward and side scatter properties suggesting they were granulocytes (Fig. 2A lower plots). The peptide-expressing cells with high scatter properties were confirmed to be granulocytes by surface marker staining with the granulocyte marker CD66c (98% of cells). The peptide expressing cells were largely negative for CD3, CD8, CD14, CD20 and CD56 expression (< 6% of these subsets) in the assay.
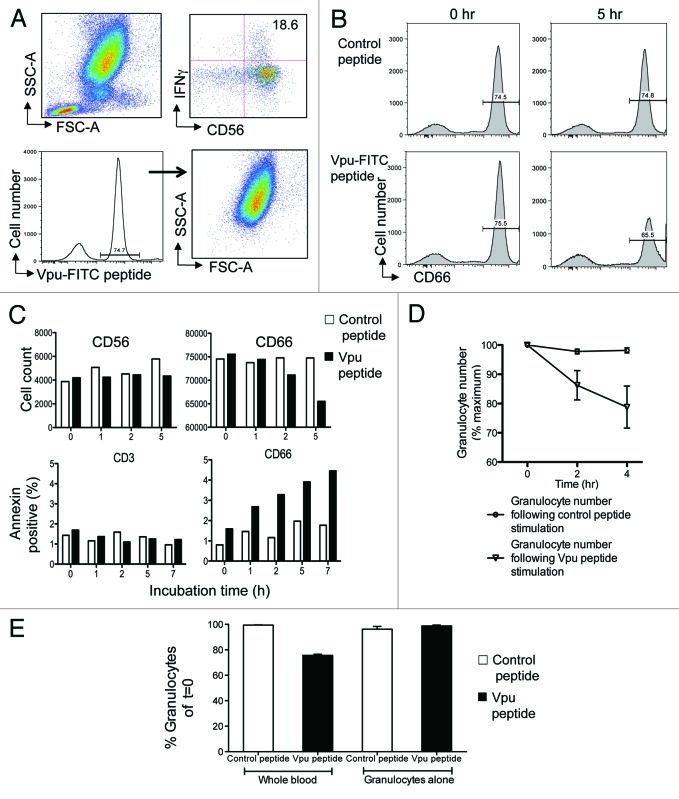
Figure 2. Granulocytes express ADCC epitopes and are targets for killing. (A) Fluorescently labeled Vpu peptide bind to granulocytes. The ADCC epitope Vpu peptide 19 (sequence EMGHHAPWDVDDL) was conjugated with FITC fluorochrome. The fluorescent Vpu peptide induced robust ADCC activity in the presence of HIV+ plasma (top right plot). Based on FSC and SCC criteria, cells expressing fluorescent peptide localized primarily to granulocytes (bottom right plot). (B) Loss of CD66c+ granulocytes (bottom right plot) occurs in the presence of HIV+ plasma during the 5 h incubation following stimulation by Vpu peptide compared with a control peptide (sequence KKFGAEVVPC) that has no known ADCC inducing ability (top right plot). (C) Granulocytes are the major cells that undergo apoptosis in the NK cell activation ADCC assay in the presence of HIV+ plasma. Decrease in CD66c+ granulocytes (top right graph) but not CD56+ NK cells (top left graph) occurred over time. Annexin V staining indicated that CD66c+ granulocytes were undergoing apoptosis (bottom right graph) compared with CD3+ T cell populations over time. Cell loss and Annexin expression in the presence of Vpu peptide (black bars) was compared with a control peptide (white bars). (D) Across 5 donors, granulocyte numbers reproducibly fell in the presence of HIV+ plasma following Vpu peptide stimulation (circles) compared with control peptide stimulation (triangles; mean and SD shown). (E) Granulocyte loss does not occur in the absence of other leukocytes. Granulocytes numbers decrease in the presence of HIV+ plasma when the ADCC assay is performed in whole blood containing NK cells (2nd bar) but not in the presence of purified granulocytes alone (4th bar). Loss of granulocytes in the presence of Vpu peptide (black bars) was compared with incubation in the presence of a control peptide (white bars).
We hypothesized that in the presence of HIV+ plasma, cells bound with Vpu ADCC peptide would be killed over time compared with cells incubated with a control peptide. Similarly, a major outcome of NK cell-mediated ADCC activation is the release of granzymes and perforin from the effector cells that lyse antigen expressing target cells, leading to a chain of intracellular events culminating in apoptosis of the target cell. Targets of cell lysis within the whole blood ADCC assay should be identifiable by the presence of apoptosis markers such as Annexin V expression. Membrane phosphatidylserine is translocated from the inner to the outer leaflet of the plasma membrane early during apoptosis.20 Annexin V binds with high affinity to surface phosphatidylserine and is a useful flow cytometry reagent to identify cells undergoing early stages of apoptosis.21
After a 5-h incubation in the presence of HIV+ plasma, CD66c+ cells bound with the Vpu-FITC peptide decreased by 10% (Fig. 2B, lower right plot) while CD66+ cells did not decrease over time when incubated with a control peptide (Fig. 2B, upper right). Reduction in CD66+ granulocytes over time (Fig. 2C, upper right bar graph) was associated with increasing expression of the apoptosis marker Annexin V (Fig. 2C, lower right bar graph). As a comparison, there was no reduction in CD56 cells numbers over time (Fig. 2C, left upper bar graph) or Annexin V expression by CD3+ cells (Fig. 2C, lower left bar graph). Further, Annexin V expression by CD2+ cells did not increase during the incubation time course while Annexin V expression by CD20+ and CD14+ cells did increase by approximately 0.5% and 1% of total number, respectively, during the incubation but not relative to control samples, suggesting some cell apoptosis during the incubation unrelated to the ADCC peptide. These results suggest that cells expressing the ADCC peptide epitope were targets for NK-mediated killing in the presence of ADCC Abs from HIV+ plasma during the assay. Replicate experiments using 5 different healthy donor NK cells were performed and we found a consistent and significant loss of peptide-expressing granulocyte numbers over time when incubated in the presence of HIV+ plasma that elicits peptide-specific ADCC (Fig. 2D).
It is important to note that granulocyte loss does not occur in the absence of other leukocytes. Granulocytes numbers decrease when the ADCC assay is performed in whole blood containing NK cells (Fig. 2E, second bar) but not in the presence of Percoll purified granulocytes alone (Fig. 2E, fourth bar). Loss of granulocytes in the presence of Vpu peptide and HIV+ plasma was compared with incubation in the presence of a control peptide, suggesting the presence of both the ADCC peptide antigen and the peptide-expressing cell (i.e., granulocytes in this case) is necessary for ADCC to occur in the presence of HIV+ plasma.
Higher ADCC activity is observed in the presence of granulocytes
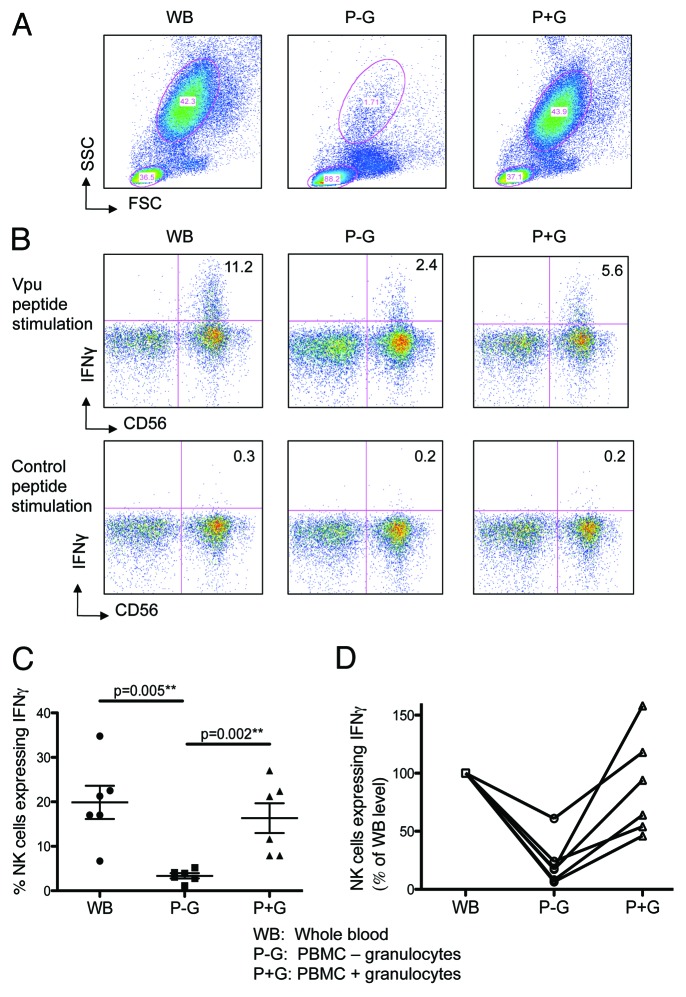
The uptake of peptides by granulocytes, the expression of the apoptosis marker Annexin V by granulocytes and the loss of granulocytes suggested that granulocytes were important in mediating peptide-specific ADCC responses. To further assess the importance of granulocytes within the peptide-specific ADCC assay, we studied the effects of granulocyte-depletion on the ADCC response. We evaluated ADCC-mediated NK cell activation in the presence of either whole blood (WB), PBMC alone (isolated over a Ficoll gradient; P-G) or PBMC preparations replenished with autologous granulocytes isolated over a Percoll gradient (P+G). We attained over 95% depletion of granulocytes, using the Ficoll gradient (Fig. 3A, middle plot), and found that the ADCC-mediated NK cell activation was significantly reduced in the absence of granulocytes (Fig. 3B, middle plot) compared with WB (Fig. 3B, left plot). The ADCC-mediated NK cell activation was significantly restored when autologous granulocytes were added back into the incubation (P+G, Fig. 3B right plot). This experiment was repeated with 5 different HIV-negative donors, and a consistent and significant restoration of ADCC activity was detected when granulocytes were replenished in the cultures (Fig. 3C). The individual donors results are graphed in Figure 3D.
Figure 3. Higher ADCC Activity is observed in the presence of granulocytes. Autologous granulocytes were added back to PBMC and assessed for their ability to restore ADCC activity. Whole blood (WB), PBMCs (isolated by ficoll gradient, P-G) and PBMCs with autologous granulocytes (isolated by percoll gradient) added back (P+G) were analyzed by flow cytometry. (A) Granulocyte population for the 3 assay conditions circled. (B) Depletion of granulocytes markedly reduces IFNγ expression by CD56 NK-cells in the presence of HIV+ plasma after stimulation with Vpu peptide (top middle plot) compared with whole blood (top left plot); this response is significantly restored by adding back granulocytes (top right plot); a control peptide showed no responses (bottom plots). (C) Across 6 donors, there is a significant decrease in IFNγ expression by NK-cells in the presence of HIV+ plasma during the ADCC assay when granulocytes were depleted (p = 0.005); this response was nearly completely restored when granulocytes were added back to the incubation (p = 0.002; Mann-Whitney) (D) Shows the same data from C normalized to NK cell activation in the whole blood culture.
HIV-1 gp140 Envelope protein binds to CD4 T cells which are targeted by ADCC
Although our results confirm that cells that express ADCC peptide antigens are targets for killing and this is associated with expression of IFNγ from CD56+ NK cells, the role of granulocytes in expressing ADCC epitopes was unexpected. Given that HIV principally infects via binding to the CD4 receptor, we therefore investigated whether similar results could be obtained using whole gp140 envelope protein. Akin to our approach with FITC-labeled peptides, we incubated whole blood from an HIV positive individual with biotinylated-gp140 and then tracked gp140 binding and cell culture kinetics using PE-conjugated streptavidin.
We first confirmed that biotinylated-gp140 effectively induced ADCC-mediated NK cell IFNγ expression in the presence of HIV+ plasma, similar to our previous work with unlabeled gp14013 (Fig. 4A). Cells from an HIV negative subject were incubated with gp140 protein in the presence of HIV negative plasma or plasma from a person with ADCC responses to gp140. We found that the number of lymphocytes co-expressing CD4 and gp140 decreased sequentially over an 8-h incubation in the presence of the plasma from the HIV-infected individual (Fig. 4B, right bar graph) while this number remained stable when the incubation proceeded in the presence of HIV negative serum (Fig. 4B, left bar graph). This reduction of lymphocytes co-expressing gp140 and CD4 was associated with an increase in Annexin V expression by cells co-expressing gp140 and CD4 (Fig. 4C). There was no decrease in granulocyte number or increase in granulocyte expression of Annexin V over 8 h in the presence of HIV-positive plasma (data not shown), as seen with peptide incubation.
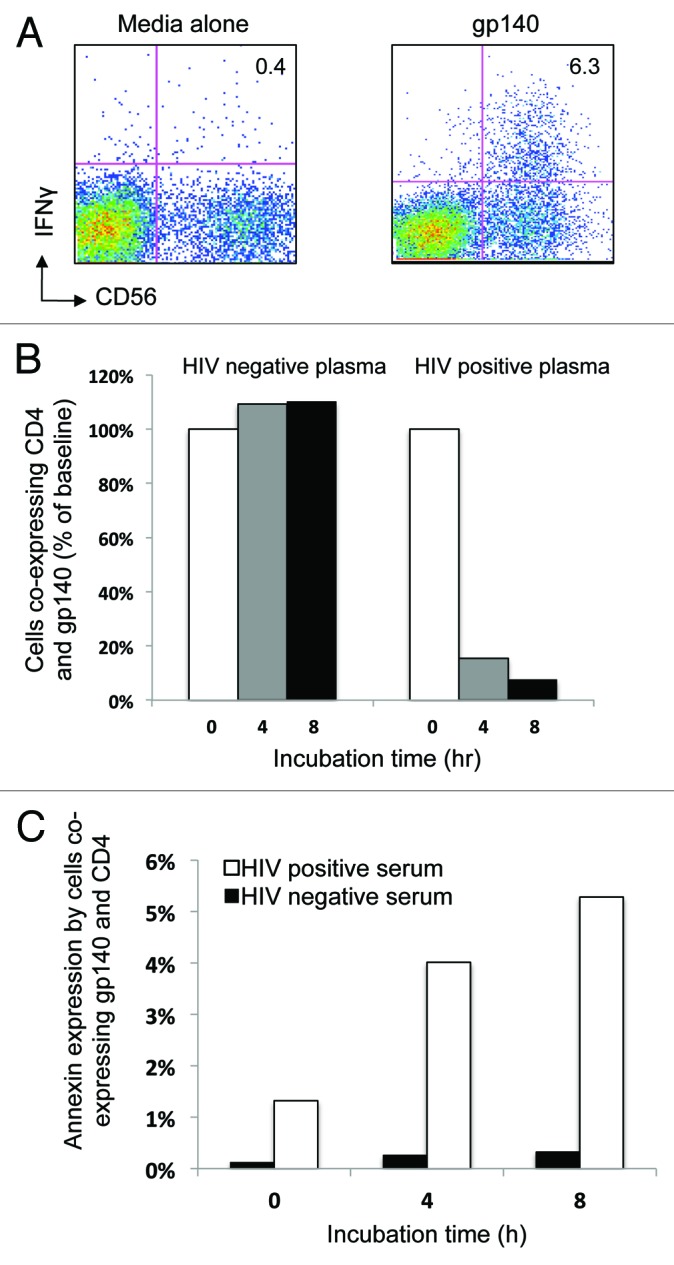
Figure 4. Cells co-expressing CD4 and HIV-1 gp140 Envelope protein reduce over time during the NK cell activation ADCC assay. (A) HIV-1 gp140 envelope protein antigen stimulation of the whole blood samples in the NK cell activation ADCC assay in the presence of HIV+ plasma induces NK cell IFNγ expression. (B) Cells co-expressing CD4 and gp140 reduced in number over time (% baseline) in the presence of HIV positive serum (right graph) but not in the presence of HIV negative serum (left graph). (C) The reduction in the number of cells co-expressing CD4+ and gp140 was associated with an increase in Annexin V expression over time in the same population when incubated in the presence of HIV+ plasma (white bars) compared with HIV- plasma (black bars).
Discussion
Since the partial success of the RV144 vaccine trial, the role of non-neutralizing antibodies (such as antibodies that mediate ADCC) in protection from HIV has gained prominence.9 ADCC antibodies have numerous potential advantages: presence at mucosal entry sites of HIV,3 the ability to target virus-infected cells, no HLA restriction and the ability to be transferred between subjects.14 We have previously demonstrated that CD56+ NK cells liberate cytokines in the presence of HIV peptide antigens, mediated by IgG, indicating an ADCC mechanism.14 We have shown that such responses are generated not only against Envelope but also against internal HIV proteins (including Vpu, Tat and Pol22), providing a potential additional benefit to ADCC-based vaccine strategies. However, the mechanism by which internal HIV proteins elicit such antibody responses was unknown. Possibilities currently under exploration include (1) parts of the virus being presented on the surface of HIV-infected cells (akin to HLA presentation of T cell epitopes) or (2) viral debris from lysed cells presented by antigen presenting cells.
Our results show that HIV-specific ADCC responses are not based on the HLA phenotype. NK cells from all 9 HIV negative donors expressed IFNγ when incubated in the presence of HIV+ plasma. It is of interest to note that the responses were not of a uniform magnitude, suggesting there may be other phenotypic features within individuals that influence these responses such as KIR phenotype23 or FcR polymorphism.24
We previously showed that HIV-antibody specific IFNγ expression by NK cells correlates with NK cell degranulation as assessed by the marker CD107a and granzyme B loss,13 however we have not previously identified the cell targets being killed by ADCC in this whole blood assay. We now show that cells bound with HIV ADCC peptide antigens expressed Annexin V and decrease in number during the assay incubation period indicating that actual killing of peptide-expressing cells is occurring. Further, we found that HIV ADCC peptides associate with CD66c+ granulocytes and that when granulocytes are depleted from the culture there is reduction in antibody-mediated NK-cell IFNγ expression. This NK cell effector function is then significantly restored when granulocytes are added back to the assay culture. It is unclear as to how granulocytes are participating in the assay. HLA presentation by granulocytes leading to activation of immune responses has been described.25 However, our data indicate that HLA restriction is not important for ADCC-mediated NK cell activity; a fact confirmed by the observation that not all HLA bearing cells take up the fluorochrome-labeled antigen. Preliminary in vitro studies with lactacystin (proteasome inhibitor) and cytochalasin D (phagocytosis inhibitor) suggest that these inhibitors do not block ADCC peptide presentation. Possible mechanisms for ADCC peptide presentation by granulocytes include FcR mediated uptake or other protein binding cell surface molecules such as integrins, a function which has mainly been studied in the context of autoimmunity.26 Other possibilities for molecules presenting short peptides by granulocytes to ADCC antibodies include selectins and scavenger receptors.27 The CD66 molecule itself could present peptides although preliminary competition assays could not confirm it (data not shown). Thus, although granulocytes permit us to define linear peptide ADCC epitopes in vitro, their role in vivo in HIV infection is not known. The large population of granulocytes lost in this 5 h ADCC assay suggests neutrophils are predominantly targeted since eosinophils and basophils make up a small proportion of granulocytes. However, further investigation of the specific types of granulocytes (neutrophils, eosinophils or basophils) playing the major role in ADCC would be interesting.
Granulocytes are not targets of HIV infection and not typically viewed as antigen-presenting cells. On the other hand, granulocytes are frequently depleted and have high levels of apoptosis during HIV and SIV infection.28-30 An initial role for neutrophils in HIV infection was postulated in the context of increased susceptibility of HIV-infected individuals to bacterial and fungal infections in neutropenic subjects.31 More recent results suggest additional roles for neutrophils given that neutrophils express several anti-viral factors, such as α-defensins and lactoferrin, which have anti-HIV-1 activity, possibly suggesting a protective role for neutrophils in HIV-1.32,33 Studies in mice have shown that neutrophils efficiently transport antigen to draining lymph nodes.34 Further, neutrophils have been shown to bind both CCR5 and CXCR4 strains of HIV-1 and efficiently transfer virus to lymphocytes.35 This would assist enhanced presentation of viral antigens to initiate or expand humoral and cellular immune responses.
Can the association of ADCC peptide epitopes with blood granulocytes be harnessed to improve HIV vaccines? We speculate that it may be possible to engineer more efficient expression of ADCC epitopes (for example, by targeting granulocytes) to stimulate high levels of immunity prior to infection and improve the immunogenicity of ADCC-inducing HIV vaccines. Recently, Duval et al. showed that a bispecific antibody incorporating a broadly reactive anti-gp41 antibody, F240, and an anti-IgA receptor (CD89) antibody is effective at directing neutrophils to destroy HIV.36 Macaques are more resistant to SHIV infection following passive transfer of neutralizing antibodies that also mediate ADCC activity.5 Vaccines that involve granulocytes to express antigens to ADCC antibodies may be less effective at controlling established HIV infection but could be useful in generating ADCC responses to prevent or ameliorate an initial exposure to HIV.
An open question remains how accurately in vitro assays of NK cell-mediated ADCC activity model the in vivo utility of HIV-specific ADCC. Our studies are limited by the use of peptide/protein pulsed cells rather that HIV-infected cells. Confocal microscopy with fluorescent-labeled peptides may be used to visualize the ADCC process. Preliminary data in our lab indicate that fluorescent peptides do associate with granulocytes but the nature of the molecular interaction is unknown.
Materials and Methods
HIV-infected subjects
HIV-infected adults with previously described HIV-specific ADCC responses were recruited to donate blood samples.14 Subjects provided informed consent and the research were conducted under the auspices of the Alfred Human Health Research and Ethics Committee. HIV infected subjects provided sodium heparin anti-coagulated blood for fresh whole blood ADCC assays and blood plasma for the ADCC assays using HIV negative donor cells.
The NK cell activation ADCC assay
The NK cell activation ADCC assay was used as previously described.14 In brief, for fresh whole blood assays, 200 μl of HIV+ whole blood (or 100 μl HIV negative donor whole blood and 100 μl HIV+ plasma) was incubated with HIV-1 Vpu19 peptide. The incubation to detect intracellular NK activation occurred in the presence of Brefeldin A (final conc. 10 µg/ml, Sigma); time course experiments to detect killing of peptide labeled cells proceeded without addition of Brefeldin A. At the end of the incubation CD56+CD3- or CD2+CD3- NK lymphocytes were studied for the expression of intracellular IFNγ. Fluorescent antibodies utilized in the NK cell activation ADCC assays were CD3 (BD Biosciences, San Jose, CA, catalog# 347344 PerCP and #641397, APC-H7), CD2 (BD #556611, FITC), CD56 (BD #555516 PE and #335791, PE-Cy7), CD66c (BD #551478, PE), IFNγ (BD #557995 Alexa700 and #554702, APC), CD8 (BD # 2217, PE), CD14 (BD # 557153, FITC), CD20 (BD # 335793, PE-Cy7) and Annexin V (BD #550474 APC).
Antigen conjugated with fluorochrome binding in NK cell activation ADCC assay
The fluorescent-labeled peptides, Vpu-FITC and control peptide were used as peptide antigens within the NK cell activation ADCC assay. Also, gp140-biotin (kindly supplied by Dr R Center) was used in HIV-1 gp140 envelope protein assays; fluorochrome identification was achieved using PE-conjugated streptavidin (BD Biosciences Cat# 554061).
Isolation of leucocytes
PBMC were separated using Ficoll density gradient centrifugation as per manufacturers’ protocol. The granulocytes were isolated from peripheral blood by density gradient centrifugation (MACS Miltenyi Biotec method) using Percoll gradient and the granulocyte purity was up to 96% as measured by CELL DYN Emerald analyzer. Briefly, the whole blood was carefully layered on top of the Percoll gradient and then centrifugation at 400xg for 30 min at 20°C in a swinging bucket rotor without brakes. Then granulocytes were carefully aspirated from the cell layer directly above the RBC and used in the assay to add back autologous granulocytes to Ficoll-separated PBMC.
HLA typing by Australian Red Cross Blood Service
HLA class I and II typing was performed on PBMC by the Australian Red Cross Blood Service using the Luminex® SSO (lab type) technique, as per manufacturer’s instructions (see www.onelambda.com).
Conclusion
In summary, we have shown that within the NK cell activation ADCC assay, cells expressing certain HIV antigens are targeted for killing by NK cells via ADCC and this mechanism is not dependent on HLA typing. These results provide a rapid and simple way of identifying ADCC epitopes in vitro. Exactly how the interactions are mediated and whether this knowledge can be utilized to improve antigen delivery in vivo and assist in vaccine design requires further investigation.
Acknowledgments
We thank staff from the Melbourne Sexual Health Centre and the patients for their generous assistance and time. The research was kindly supported by NHMRC award 510448, ARC award LP0991498, the ACH2, the RACP, Ramiciotti Foundation and NIH award R21AI081541.
Glossary
Abbreviations:
- ADCC
antibody-dependent cellular cytotoxicity
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23446
References
- 1.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–73. [PubMed] [Google Scholar]
- 2.Ljunggren K, Chiodi F, Biberfeld G, Norrby E, Jondal M, Fenyö EM. Lack of cross-reaction in antibody-dependent cellular cytotoxicity between human immunodeficiency virus (HIV) and HIV-related West African strains. J Immunol. 1988;140:602–5. [PubMed] [Google Scholar]
- 3.Nag P, Kim J, Sapiega V, Landay AL, Bremer JW, Mestecky J, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis. 2004;190:1970–8. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rook AH, Lane HC, Folks T, McCoy S, Alter H, Fauci AS. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987;138:1064–7. [PubMed] [Google Scholar]
- 5.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 6.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–27. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 8.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Jr, Carville A, et al. ADCC Develops Over Time during Persistent Infection with Live-Attenuated SIV and Is Associated with Complete Protection against SIVmac251 Challenge. PLoS Pathog. 2012;8:1–12. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, et al. Thai AIDS Vaccine Evaluation Group, Thailand Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2522–9. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 12.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–10. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 14.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–9. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A. 2011;108:7505–10. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58:127–31. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thobakgale CF, Fadda L, Lane K, Toth I, Pereyra F, Bazner S, et al. Frequent and strong antibody-mediated natural killer cell activation in response to HIV-1 Env in individuals with chronic HIV-1 infection. J Virol. 2012;86:6986–93. doi: 10.1128/JVI.00569-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiemessen CT, Shalekoff S, Meddows-Taylor S, Schramm DB, Papathanasopoulos MA, Gray GE, et al. Cutting Edge: Unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–8. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Román VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Reutelingsperger CP, van Heerde WL. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell Mol Life Sci. 1997;53:527–32. doi: 10.1007/s000180050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology. 2011;412:110–6. doi: 10.1016/j.virol.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons MS, Wren L, Isitman G, Navis M, Stratov I, Bernard NF, et al. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol. 2012;86:4488–95. doi: 10.1128/JVI.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leslie RG. Immunoglobulin and soluble immune complex binding to phagocyte Fc receptors. Biochem Soc Trans. 1984;12:743–6. doi: 10.1042/bst0120743. [DOI] [PubMed] [Google Scholar]
- 25.Reali E, Guerrini R, Moretti S, Spisani S, Lanza F, Tomatis R, et al. Polymorphonuclear neutrophils pulsed with synthetic peptides efficiently activate memory cytotoxic T lymphocytes. J Leukoc Biol. 1996;60:207–13. doi: 10.1002/jlb.60.2.207. [DOI] [PubMed] [Google Scholar]
- 26.Németh T, Mócsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 27.van der Vaart M, Spaink HP, Meijer AH. Pathogen recognition and activation of the innate immune response in zebrafish. Adv Hematol. 2012;2012:159807. doi: 10.1155/2012/159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elbim C, Monceaux V, François S, Hurtrel B, Gougerot-Pocidalo MA, Estaquier J. Increased neutrophil apoptosis in chronically SIV-infected macaques. Retrovirology. 2009;6:29–34. doi: 10.1186/1742-4690-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuritzkes DR. Neutropenia, neutrophil dysfunction, and bacterial infection in patients with human immunodeficiency virus disease: the role of granulocyte colony-stimulating factor. Clin Infect Dis. 2000;30:256–60. doi: 10.1086/313642. [DOI] [PubMed] [Google Scholar]
- 30.Salmen S, Montes H, Soyano A, Hernández D, Berrueta L. Mechanisms of neutrophil death in human immunodeficiency virus-infected patients: role of reactive oxygen species, caspases and map kinase pathways. Clin Exp Immunol. 2007;150:539–45. doi: 10.1111/j.1365-2249.2007.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermans P. HIV disease-related neutropenia: an independent risk factor for severe infections. AIDS. 1999;13(Suppl 2):S11–7. [PubMed] [Google Scholar]
- 32.Guo CJ, Tan N, Song L, Douglas SD, Ho WZ. Alpha-defensins inhibit HIV infection of macrophages through upregulation of CC-chemokines. AIDS. 2004;18:1217–8. doi: 10.1097/00002030-200405210-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saidi H, Eslahpazir J, Carbonneil C, Carthagena L, Requena M, Nassreddine N, et al. Differential modulation of human lactoferrin activity against both R5 and X4-HIV-1 adsorption on epithelial cells and dendritic cells by natural antibodies. J Immunol. 2006;177:5540–9. doi: 10.4049/jimmunol.177.8.5540. [DOI] [PubMed] [Google Scholar]
- 34.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, et al. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 35.Olinger GG, Saifuddin M, Spear GT. CD4-Negative cells bind human immunodeficiency virus type 1 and efficiently transfer virus to T cells. J Virol. 2000;74:8550–7. doi: 10.1128/JVI.74.18.8550-8557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duval M, Posner MR, Cavacini LA. A bispecific antibody composed of a nonneutralizing antibody to the gp41 immunodominant region and an anti-CD89 antibody directs broad human immunodeficiency virus destruction by neutrophils. J Virol. 2008;82:4671–4. doi: 10.1128/JVI.02499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]