Breast cancer is second only to non-melanoma skin cancers as the most prevalent and lethal malignancy among women worldwide.1 Upon diagnosis, cases are clinically stratified based upon their estrogen-, progesterone-, and HER2-receptor status. Together, these factors are predictive of treatment response and prognosis. Although effective therapeutic options exist for hormone receptor-positive breast cancer patients, those with triple receptor-negative breast cancers (TNBCs) have the poorest prognoses, and no targeted therapies are currently available.
Transcriptional profiling of TNBC-subtype tumors has revealed striking heterogeneity, but has also led to the identification of molecularly defined clusters.2 Several high-profile sequencing studies (e.g., the TCGA) have shown that while TNBCs share relatively few oncogenic mutations, TP53 is frequently mutated throughout this subtype.3,4 For this reason, despite large-scale sequencing and transcriptional profiling studies, few unique driver genes have been identified that would serve as effective TNBC subtype-specific molecular targets.
The advent of shRNA libraries has enabled new approaches to large-scale transcriptomic and genomic screening, which have identified that loss of expression of putative tumor suppressor phosphatases (INPP4B and PTPN12) occurs across a large fraction of TNBCs. Unfortunately, these approaches have yet to reveal easily, therapeutically targetable tumor suppressors for clinical use.
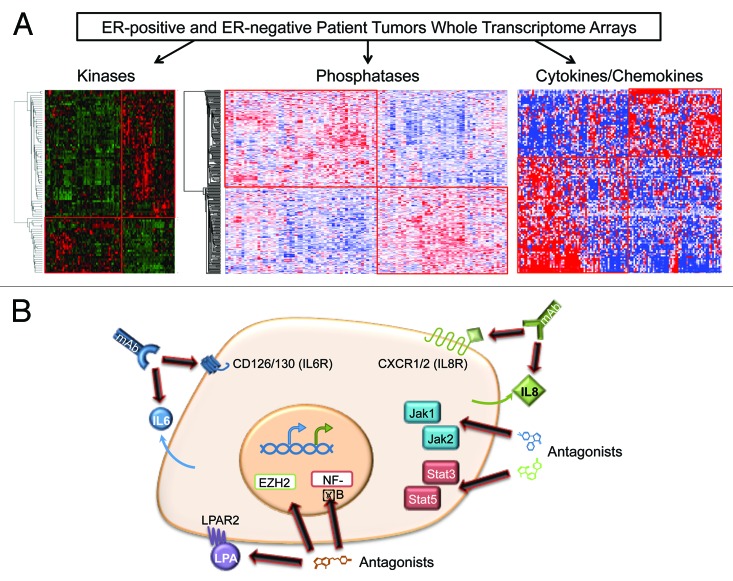
To identify specific therapeutic targets, investigators have examined the expression and activation of functional sets of genes or proteins using genomic profiling data. In 2009, we discovered a set of kinases overexpressed in ER-negative breast cancer, which defined clinically relevant patient stratifications and identified new therapeutic targets currently under examination. This strategy of combining transcriptome data with known gene function and patient outcome data has been further expanded to include oncogenic/tumor-suppressing phosphatases and immune-related signaling genes in ER-negative breast cancers (Fig. 1A).5
Figure 1. Identification of novel therapeutic targets in estrogen receptor-negative breast cancers and potential strategies for treatment. (A) A strategy to identify candidate targets: breast cancer patients are transcriptionally profiled using whole-genome microarrays, with an overselection bias to generate similar numbers of ER(−) and ER(+) patients. The transcriptional landscapes are then broken down into functionally related genes for further analysis, including subsets of kinases, phosphatases, and inflammatory genes. (B) Potential avenues for therapeutic targeting of ER(−) stem-like breast cancer cells. A combination of commercially available monoclonal antibodies recognizing IL6/IL8 or their receptors or pharmacologic inhibitors is required to maximally block cancer cell growth. Additional targeting access points in the IL6/IL8 pathways include LPA, JAK/STAT, EZH2, or NFκB.
In a manuscript recently published in Cancer Research,6 we described a set of cytokine/chemokines critical for TNBC growth, including a core set of 10 pro-inflammatory factors essential for TNBC tumorigenicity. Multiple lines of in silico and in vitro evidence revealed that loss of both IL-6 and IL-8 inhibits tumor cell growth more than knockdown of either protein alone. Mouse xenograft studies support these results, demonstrating more effective suppression of TNBC cell growth with dual IL-6/IL-8 knockdown. Early-phase clinical trials targeting IL-6 and IL-8 or their cognate receptors in breast cancer are currently underway (see ClinicalTrials.gov identifier NCT00433446 and UMCC 2011.079). However, recent data indicate the promise of combinatorial inhibitor trials capable of targeting multiple inflammatory pathways. While IL-6 and IL-8 are classically thought to function through paracrine mechanisms, these new results demonstrate that in tumor growth IL-6 and IL-8 function primarily through autocrine signaling.
This work supports recent findings that increased IL-6 and IL-8 autocrine and paracrine signaling promotes tumor stem cell propagation, epithelial-to-mesenchymal transition, and a metastatic phenotype in breast cancer stem cells that predominantly express IL-6 and IL-8 receptors.7,8 Our results show that TNBC patients with high IL-6- and IL-8-expressing tumors have worse prognoses. Taken together, these suggest a TNBC-specific regulatory feedback program incorporating the LPA-LPAR2-EZH2/NFκB signaling axis and the JAK/STAT and mitogenic activation pathways. These novel findings demonstrate the potential of combined IL-6/IL-8-inhibition or the targeting of critical signaling nodes (cytokines and JAK/STAT3) for the treatment of TNBC (Fig. 1B). Our current focus is on defining the mechanisms involved with IL-6/IL-8 overexpression that also implicates EZH2 and NFκB in the process.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25871
References
- 1.Ferlay J, et al. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Curtis C, et al. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SP, et al. Nature. 2012;486:395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speers C, et al. Clin Cancer Res. 2009;15:6327–40. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman ZC, et al. Cancer Res. 2013;73:3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marotta LL, et al. J Clin Invest. 2011;121:2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korkaya H, et al. Mol Cell. 2012;47:570–84. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]