Figure 3. CDH23 peptide cytochemistry and effects of CDH23 peptides on vibration sensitivity in Nematostella vectensis.
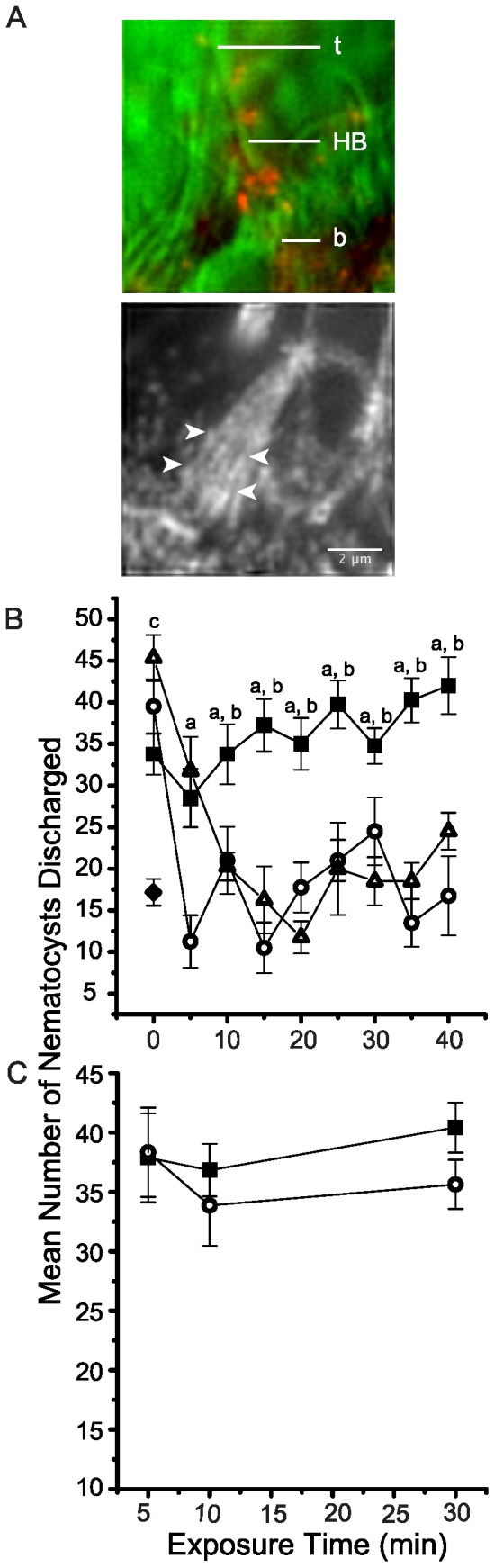
(A) Fluorescently tagged CDH23 peptides label stereocilia near the base of a hair bundle with punctate fluorescence. The upper micrograph depicts a merged image of a hair bundle shown in transmitted light using oblique contrast (assigned green) and epifluorescence microscopy for CDH23 peptide-FITC cytochemistry (assigned red). The tip (t) and base (b) of the hair bundle (HB) are indicated. The lower micrograph depicts immunocytochemistry for TRPN1. It is intended to show the morphology of stereocilia in the hair bundle. White arrowheads indicate the height of the tips of several small diameter stereocilia. Scale bar = 2 µm. (B) Vibration sensitivity was tested at intervals after adding 0.1 nM CDH23 peptide (open triangles, final concentration) or 10 nM CDH23 peptide (open circles, final concentration) to the seawater containing intact anemones. The mean number of nematocysts counted per field of view (±SEM, n = 6) is plotted for the experimental animals as well as for untreated, vibrating controls (closed squares) and non-vibrating controls (closed diamond). aSignificant difference between mean nematocyst discharge for the 10 nM CDH23 peptide treated animals and untreated, vibrating controls. bSignificant difference between mean nematocyst discharge for the 0.1 nM CDH23 peptide treated animals and untreated, vibrating controls. cSignificant difference between non-vibrating controls and the other three treatments at time 0. (C) Vibration sensitivity was tested at intervals after adding 10 nM harmonin peptide (open circles, final concentration) as a peptide-loading control. The mean number of nematocysts discharged is plotted (±SEM, n = 6–8) for untreated vibrating controls (closed squares) and for animals exposed to the harmonin peptide (open circles). Asterisks indicate significant differences in mean nematocyst discharge (p<0.05).
