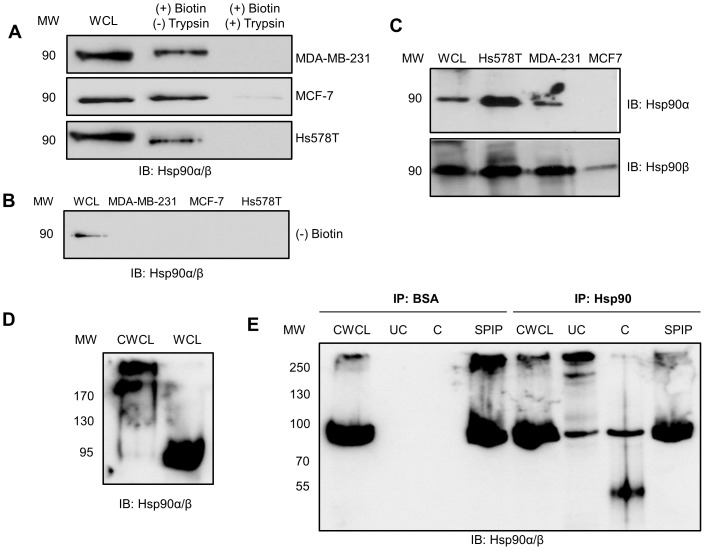
Figure 1. Hsp90 is present in a putative complex on the plasma membrane of MDA-MB-231 cells.
(A) Confluent MDA-MB-231, MCF-7 and Hs578T cells were subject to (+) biotin and (±) trypsin treatment. Biotinylated proteins were purified and equal amounts of protein resolved by SDS-PAGE and probed for Hsp90α/β by immunoblotting. Whole cell lysates (WCL) are shown as a reference. (B) MDA-MB-231, MCF-7 and Hs578T cells treated without NHS-biotin were probed for Hsp90α/β by immunoblotting and showed no Hsp90 in the purified fraction. WCL of Hs578T cells were used as a positive control for immunoblotting. (C) Biotinylated fractions from equal numbers of Hs578T, MDA-MB-231 or MCF-7 cells were probed for the presence of either Hsp90α or Hsp90β using isoform specific antibodies. The WCL from Hs578T cells was used as a positive control for immunoblotting. (D) Surface proteins of MDA-MB-231 cells were chemically crosslinked with BS3 reagent and the crosslinked whole cell lysate (CWCL) and whole cell lysate (WCL) probed for Hsp90α/β by immunoblotting. (E) Surface proteins of MDA-MB-231 cells were chemically crosslinked with the thiol-cleavable DTSSP reagent and cell lysates immunoprecipitated with either BSA (IP: BSA; Negative control) or Hsp90α/β primary antibody (IP: Hsp90). The crosslinked whole cell lysate (CWCL), uncleaved (UC) and cleaved (C) immunoprecipitates, and supernatant post immunoprecipitation (SPIP) were probed for Hsp90α/β by immunoblotting. The data shown are representative of results obtained from triplicate experiments in all cases.