Fig. 4.
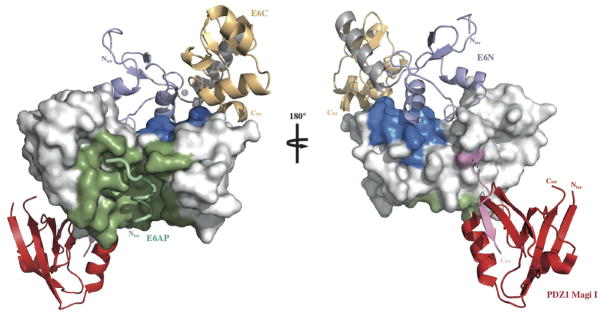
Mapping HPV16 E6 functional regions. The different binding sites of HPV16 E6 map to distinct regions of the protein’s solvent accessible surface. LxxLL binding and E6 self-association residues are colored green and blue respectively. The position of the second E6 molecule shown in the ribbon representation was modeled based on the geometry of the E6N homodimer interface (2LJY.pdb). The C-terminal PDZ binding motif (pink) is disordered in the NMR structure of the isolated HPV16 E6C domain and adopts a β-strand conformation upon binding to the PDZ1 of Magi I (red) (2KPL.pdb). The relative orientation of E6 and the PDZ domain shown is arbitrary. Surfaces colored white are potentially available for binding to p53. See also Supplemental Fig. 9.
