Abstract
Background:
The purpose of the study was to determine the age at which initiation of specific subcutaneous immunotherapy (SCIT) becomes more cost-effective than continued lifetime intranasal steroid (NS) therapy in the treatment of allergic rhinitis, with the use of a decision analysis model.
Methods:
A Markov decision analysis model was created for this study. Economic analyses were performed to identify “break-even” points in the treatment of allergic rhinitis with the use of SCIT and NS. Efficacy rates for therapy and cost data were collected from the published literature. Models in which there was only incomplete improvement while receiving SCIT were also evaluated for economic break-even points. The primary perspective of the study was societal.
Results:
Multiple break-even point curves were obtained corresponding to various clinical scenarios. For patients with seasonal allergic rhinitis requiring NS (i.e., fluticasone) 6 months per year, the age at which initiation of SCIT provides long-term direct cost advantage is less than 41 years. For patients with perennial rhinitis symptoms requiring year-round NS, the cut-off age for SCIT cost-effectiveness increases to 60 years. Hypothetical subjects who require continued NS treatment (50% reduction of previous dosage) while receiving SCIT also display break-even points, whereby it is economically advantageous to consider allergy referral and SCIT, dependent on the cost of the NS prescribed.
Conclusion:
The age at which SCIT provides economic advantages over NS in the treatment of allergic rhinitis depends on multiple clinical factors. Decision analysis models can assist the physician in accounting for these factors and customize patient counseling with regard to treatment options.
Cost control currently drives the climate in all areas of medical practice. Scrutiny of health-care utilization and cost comparisons has become the norm with regard to physician compensation and management.1–5 There is now a heightened awareness of increased health care costs and the driving forces behind them. Many in the field of medicine are reexamining standard approaches to the management of conditions, based on the efficacy of any given intervention and the economics/cost involved.6–9
Pharmacoeconomics continues to play a pivotal role in determining cost-benefit analysis.10–12 This field has proven to be instrumental in other aspects of medicine and surgery with regard to new interventions and medications. In the field of allergy, much work has been done with regard to intervention studies in allergic rhinitis (AR) and asthma and cost-effectiveness of medications.13–15 Furthermore, numerous studies have been performed in Europe showing a significant cost savings for patients with AR treated with subcutaneous immunotherapy (SCIT).16–20 However, according to a recent editorial by Lockey and Hankin,21 only 4 studies have been performed in the United States that pertain to the health economics of SCIT. Three of the four studies were designed to evaluate retrospective claims data, whereas the fourth study was a hypothetical modeling system. Despite variations and robustness in design, each of these studies has shown significant cost savings of SCIT.22–25
Visits for AR to primary care specialists, otolaryngologists, and allergy/immunologists are increasing yearly.26 The cost burden to society has grown precipitously in recent years, with direct costs reaching $7.3 billion and indirect costs soaring to $4.28 billion.27 It is clear that patients with this disease are facing difficult problems with regard to their health, including morbidity concerns, reduced work productivity, and lost days at school.
According to the latest practice parameter, intranasal steroids (NS) are the gold-standard treatment for this disease,28 but this treatment is not disease-modifying, and, because symptoms recur shortly after they are discontinued, they must be taken for a lifetime. However, in seasonal AR, these medications do not have to be given year-round and are effective if provided only during periods of relevant allergen exposure.29–31 SCIT, on the other hand, is disease-modifying and can induce long-term tolerance after 3–5 years of therapy.32–37 Despite this, on the presumption of cost and efficacy, immunotherapy is usually reserved and considered appropriate and recommended only for patients with AR who remain symptomatic with allergen avoidance and appropriate medical therapy.32 In contrast, after considering seasonality of symptoms and the necessary duration of treatments, we wanted to determine if immunotherapy would be a more cost-effective management solution in patients with rhinitis who are well controlled with intranasal steroids. In reality, patients who receive efficacious treatment with NS may never present to a specialist for consideration of allergen immunotherapy. Therefore, we were interested in cost analysis of these patients with regard to NS or SCIT. Specifically, we posited that there would be an age at which immunotherapy was a better economic alternative to life-long intranasal steroids. A multitude of variables exist for this question, including age at presentation, duration of symptoms during the year, the efficacy of the given therapy, and the number of sensitized allergens for which an individual requires SCIT. Therefore, we determined that the most efficient way to evaluate this question would be through decision analysis with the use of Markov modeling.
METHODS
With the use of TreeAge Pro 2012 software (TreeAge Pro, Williamstown, MA), we developed a Markov model to analyze the cost differences between the use of intranasal steroids for life versus SCIT for 3–5 years. In this model, a decision tree evaluates multiple cycles (e.g., years in a human lifespan) with each cycle passing through a decision node and the results independent of the previous cycle.
To simulate medical decision-making, the Markov model must closely approximate real-life clinical decisions. The model (Fig. 1) starts with a patient who presents with rhinitis and has shown response to a nasal steroid. The clinician must then make a decision (decision node): continue treatment with a nasal steroid or send the patient for allergy testing. After each decision node (nasal steroid or allergy testing), there is a Markov node. The Markov node is followed by various Markov states. In this clinical scenario, the Markov states are baseline, NS, allergy-negative continue NS (allergy testing was negative, continue NS), immunotherapy fail continue NS (SCIT fails to improve symptoms, continue NS), immunotherapy for 3 years (or 5 years), and death. The theoretical patient progresses through the various Markov states on the way to death at 80 years, with the time spent (and cost incurred) in a state dependent on variables denoted at each chance node. The values for the variables are listed under the chance node; the values were obtained from the literature.
Figure 1.
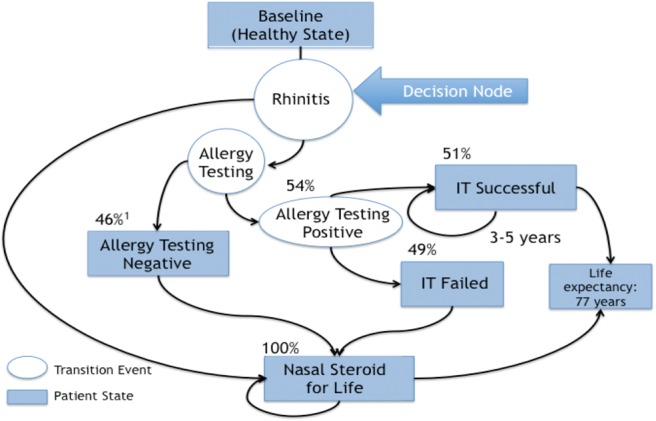
Markov model and assumptions for hypothetical computer analysis. Each circle represents a decision node, which requires medical decisions. Represented are the assumptions made for each of the decision nodes. Boxes represent patient states in which no further medical decisions are required.
If a patient is given the nasal steroid route, they first pass through the baseline Markov state. This enables tunneling whereby the age of AR diagnosis can be varied. Once the age of diagnosis is reached, they transfer to the nasal steroid state. Because the allergy test rate is 0 in the nasal steroid limb, all patients remain in the nasal steroid state until they die at age 80 years, incurring a yearly cost of a nasal steroid.
If a patient progresses to the allergy testing limb at the decision node, they will remain in baseline state until the age they are diagnosed with AR, then transition to the nasal steroid state in which they immediately get tested for allergies. This incurs a one-time cost of allergy testing. If results are negative, the patient continues to use nasal steroids until death. If test results are positive, immunotherapy is attempted for 1 year, and, if successful, continues for 2 more years, incurring the costs of SCIT. If the patient fails SCIT, they resume nasal steroids, having incurred the costs of allergy testing and the first year of SCIT before continuing to incur the costs of yearly nasal steroids.
The Markov model requires the input of variables including the age at which a change in therapies was considered; the third-party payer reimbursement for immunotherapy; drug costs for nasal steroids, allergic evaluation costs, and immunotherapy efficacy; and the probability of an individual patient being referred for an AR evaluation to have positive skin test results.
For the purposes of this model, we assumed that there would be complete compliance with either treatment measure and that nasal steroids were 100% effective for the study population, given our clinical question (e.g., cost of SCIT in patients in whom nasal steroids are effective). The cost of generic fluticasone was set on the basis of 2010 Micromedex values ($250/year). We chose this nasal steroid because it was the most inexpensive choice, and any alternatives would only serve to further increase costs in this subgroup of analysis. However, we also conducted 2-way sensitivity analysis, which includes evaluation of yearly cost versus the age of diagnosis with AR. With the use of this model, we were able to predict the economic “break-even” points for the most commonly prescribed nasal steroids on the market, on the basis of their 2010 Micromedex values. We based our allergy office evaluation on CPT code 99244 and limited the skin testing evaluation to 20 prick skin tests with a CPT code 95004. Reimbursement was based on third-party payers and estimate 190% of 2012 Medicare contracted rates at the University of Virginia. However, these costs will vary, depending on the state and insurance carrier.
On the basis of previous research, we assumed that 54% of the population at large would have positive skin test results38 and that SCIT would only be efficacious in 51% of the population.39 We assumed that all SCIT patients would receive the recommended doses proven to provide clinical benefit. We also posited that SCIT would initially be given at weekly intervals until maintenance concentrations were achieved and would occur at 6 months, after which, as per practice parameter recommendations, SCIT would be continued at monthly intervals. As noted, we modeled that SCIT would be stopped after 1 year if not effective. Last, if successful, our model required that successful therapy would continue for a total of only either 3 or 5 years, as recommended in the allergy practice parameters.28
We recognized that not all patients who require immunotherapy are able to completely stop medications, including NS. For this reason, we modified the SCIT arm, and, in a secondary analysis, we evaluated the “break-even” point, whereby patients would continue NS treatment but at a 50% reduction from levels used before SCIT during SCIT therapy.33 Furthermore, it is plausible that patients receiving SCIT might require a second therapeutic intervention with this modality. Therefore, we also modified the analysis to evaluate the number of years required between each treatment schedule for SCIT to remain economically advantageous. For this analysis, we assumed that the patient received generic fluticasone and had private insurance with equivalent reimbursement (190% of Medicare). It also included the cost of repeat skin testing, which probably would be appropriate to exclude new allergic sensitizations.
RESULTS
When considering cost-effectiveness of either intranasal steroids or SCIT, the age of the patient at referral to the specialist for consideration of SCIT, life expectancy, the number of months per annum during which NS are required, the cost of NS, the number of vials required to treat the sensitized allergens, and the duration of treatment with SCIT (3 or 5 years) are important variables. Consideration for the efficacy of the interventions and the need for a specialty evaluation also add to the cost. Our model considered all of these variables and created cost analysis of intranasal steroids and SCIT, dependent on the age of the patient, the number of months the patient required intranasal steroids, and the duration of SCIT.
Fig. 2 A illustrates the “break-even” age whereby it becomes more cost-effective to consider allergy testing and SCIT in a subject in whom NS are effectively controlling their disease. This analysis considers third-party reimbursement for generic fluticasone and 1 vial of SCIT, as well as the age of the patient at referral and how many months per year the patient requires intranasal steroids. Ultimately, our model as illustrated in Fig. 2 A allows for cost analysis of nasal steroids compared with SCIT for any given age. For example, we could pose hypothetical scenarios such as in the case of a 30-year-old patient who requires intranasal steroids 6 months of the year. In this example, the graph details that it is more cost-effective to switch to 1 vial of SCIT for either 3 or 5 years of therapy. The same patient who only requires 3 months of therapy with intranasal steroids should remain on these medications, if cost analysis is the only factor considered. Our hypothetical patient would require NS for either 5 months of the year (3 years of SCIT) or 6 months of the year (5 years of SCIT) before SCIT becomes economically advantageous. In contrast, year-round intranasal steroids are only more cost-effective than 1 vial of SCIT after the age of 60 years.
Figure 2.
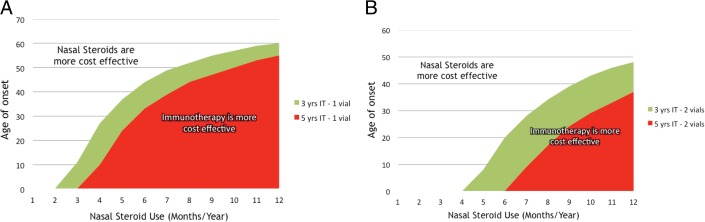
Evaluation of the “break-even” age at which continuing NS is no longer cost-effective. Third-party reimbursement for generic fluticasone and SCIT when considering both the age of the patient and how many months per year the patient requires intranasal steroids. (A) One vial of SCIT for 3 (green) or 5 years (red). (B) Two vials of SCIT for 3 (green) or 5 years (red).
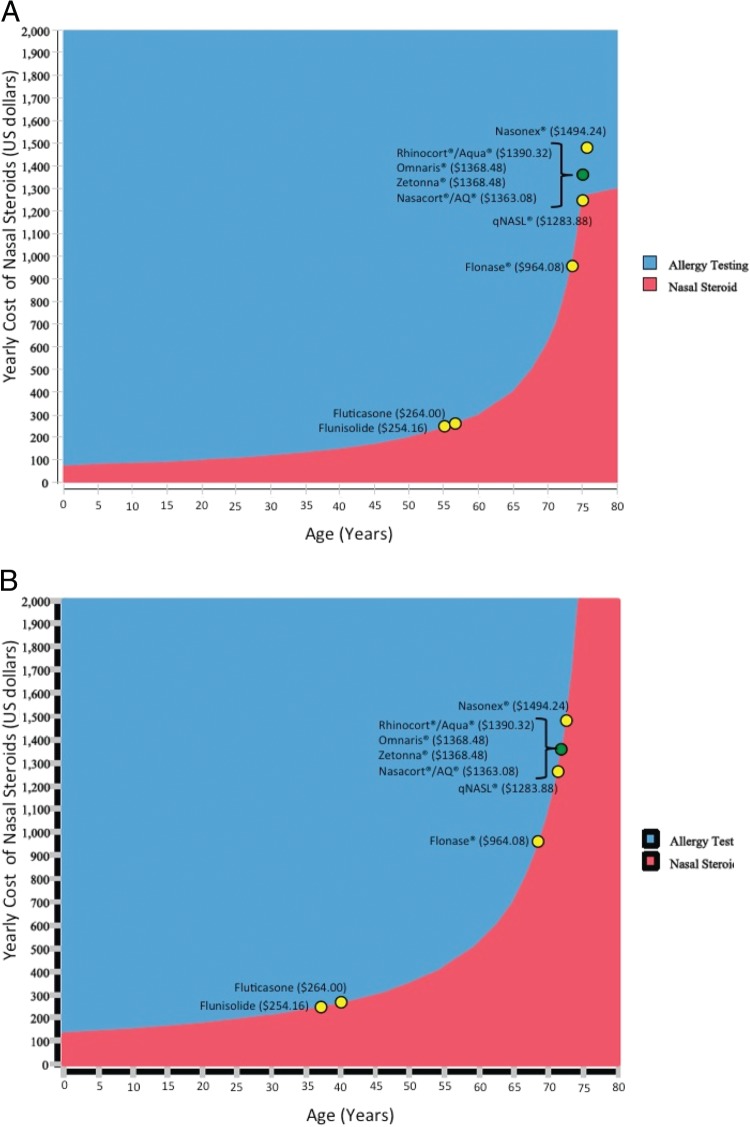
Fig. 2 B considers third-party reimbursement for generic fluticasone and 2 vials of SCIT for either 3 or 5 years. For 2 vials of SCIT, in the scenario in which nasal steroids are required year-round, intranasal steroids become more cost-effective after the age of 48 years. On the basis of these data, it is obvious that the higher the cost of the steroid, the more the curve shifts in favor of SCIT, which is exactly what we see when other steroids are included in our analysis (Fig. 3, A and B).
Figure 3.
Two-way sensitivity analyses of the yearly costs incurred with NS versus age at which SCIT and skin testing for AR is considered. Yellow circles represent the economic “break-even” points, in which the cost of allergy testing and 1 round of SCIT for 3 years begins to overshadow the yearly cost of NS. (A) One vial of SCIT. (B) Two vials of SCIT. (C) One vial of SCIT, but the patient continues to receive NS at 50% of dose before SCIT.
Next, we performed an analysis of 1 vial (Fig. 3 A) or 2 vials (Fig. 3 B) of SCIT when the yearly costs incurred from either SCIT or NS versus the age of the subject at referral are considered. In these figures, the x-axis identifies the age at which NS become more cost-effective, and the y-axis indicates the yearly cost of NS. The 2-way sensitivity analysis generated economic “break-even” ages (the yellow dots) at which it remains less expensive to remain on NS for life than sending the subject for allergy testing and consideration of SCIT. As would be expected, NS that remain on patent or name brand are more expensive and skew the cost-savings analysis more and more toward sending the patient for allergy evaluation and starting SCIT at an older age.
We modified the SCIT arm of the study to include patients who improve on SCIT but still require NS at a 50% reduction of doses needed before starting immunotherapy. With the use of this format, the cost-analysis curve does shift, but only ∼4–5 years to the left (younger) if considering only 1 vial of immunotherapy. However, an economic “break-even” point remains for all of the medications evaluated (Fig. 3 C). Finally, we further modified the analysis to evaluate how long SCIT would need to be efficacious before another round of therapy could be implemented and remain economically advantageous. According to our model, allergy shots remain cost-effective compared with NS for life if SCIT is provided 3 or 5 years and remains effective for 9.23 (1 vial of SCIT) or 12.69 (2 vials of SCIT) years, respectively, before another round of shots (data not shown).
DISCUSSION
With the use of our Markov model, we were able to project costs for both SCIT and NS, dependent on the data available to us, and then apply these yearly costs to hypothetical patients. On the basis of this modeling system, SCIT is more cost-effective than NS, depending on age of presentation of the patient to a physician qualified to perform skin testing, the number of sensitized allergens (number of vials), and the seasonality of symptoms (number of months/year for which NS would be used). We attempted to account for this last variable by allowing treatment with NS only during times of symptoms.
Much like the previous US studies of SCIT cost-effectiveness,22–25 our study also proves the economic benefit of this therapy for most patients. Our study is unique for several reasons. Intranasal steroids are the gold-standard treatment of AR, and we are the first to evaluate the pharmacoeconomics of continuing this therapy in patients in whom these medications are working versus changing to SCIT. Also, unlike previous hypothetical modeling systems, with the use of advanced computer software, we are able to manipulate the input to make it relevant for any of the variables.
We chose to focus on patients who were already doing well on intranasal steroids because these are the patients who probably will never be sent to specialists or be considered for SCIT. In other words, most patients in whom nasal steroids are effective would continue these medications without consideration for any alternative treatments. For this reason, our model also accounted for the cost of skin testing and a specialist visit, which might otherwise have been avoided. Alternatively, variables such as patient compliance and efficacy of the intervention could be inadequately appreciated. However, ultimately, our study design does not allow for deciding whether or not either intranasal steroids or SCIT are going to be effective.
We were careful to consider efficacy of SCIT in our model because we did not want to assume 100% effectiveness in an intervention that the hypothetical patients had not attempted at presentation. Therefore, we used a study containing modest efficacy for SCIT: 51%.39 This figure was based on a meta-analysis of SCIT performed by Ross et al39 that evaluated 16 randomized, prospective, single- or double-blind placebo-controlled trials and determined that SCIT was effective at a rate of 51%. The use of this conservative figure allowed us to remove any bias from our analyses that might favor SCIT.
In addition, it is known that 54% of the US population has skin test results positive to common allergens.38 The prevalence of allergic disease in the United States is much lower, currently estimated to be 7–10% of the population,40 consistent with the recognition that allergy skin testing does not often predict symptoms on natural exposure.41 For this reason, the positive skin test for allergic disease must always be considered and correlated with the history, a luxury that hypothetical models do not afford. However, in our model, the theoretical patient population included only patients with rhinitis who were doing well with NS. In this particular population, the actual rate of positive skin tests is likely to be much higher. Therefore, the conservative figure of 54% positive allergy skin tests prevents skewing of the data toward an economic advantage to SCIT.
We assumed that allergen immunotherapy would only be provided with recommended doses that include weekly injections during the build-up phase and monthly injections for a maximum of 5 years, on the basis of allergy SCIT guidelines.28 We assumed that SCIT would be administered at the doses recommended by these guidelines, which also formed the basis for our assumption that SCIT would provide ∼51% efficacy.39 Therapy extending beyond this time period would obviously incur much greater cost, thereby making it more likely that intranasal steroids for life would be more cost-effective. We also assumed that 3–5 years of SCIT would be effective for life. Regarding this last assumption, it is important to note that well performed longitudinal studies of SCIT have not evaluated patients after more than 12 years34,35,42–44; therefore it is difficult to conclude that this will prove to be true. For example, on the basis of current research involving sublingual immunotherapy, there appears to be a window of improvement of only ∼10 years after stopping this intervention. Unfortunately, these patients in general were not improved as much as those receiving SCIT; therefore it may not be appropriate to extend this observation regarding the limited duration of efficacy of sublingual immunotherapy to SCIT.45 However, it is plausible that a patient may respond to SCIT initially, and, once the therapy is discontinued after 3 or 5 years, symptoms may resume. To account for this example, we have evaluated the number of years SCIT must provide symptomatic relief without other medications before another round of SCIT is initiated for this treatment to remain cost-effective. According to our model and assumptions (generic fluticasone, repeat skin testing, and private insurance), allergy shots remain cost-effective compared with NS for life if SCIT is provided 3 or 5 years and remains effective for 9.23 or 12.69 years, respectively, before another round of shots. If other more costly NS are used, SCIT therapy becomes increasingly more cost-effective in comparison and the number of years between rounds of shots becomes fewer (data not shown).
Furthermore, we included an evaluation of hypothetical subjects who complete SCIT but remain on NS at a 50% reduction of their dosage before SCIT. These results are outlined in Fig. 3 C, in which the yellow dots represent the age at which NS for life become more cost-effective than referral for allergy testing and SCIT. As it should, the curve shifts to younger ages to remain on NS, but only slightly (∼4 years) (Fig. 3 D). These data were unexpected because we had assumed that the added costs of continuing NS at 50% of the previous dose would eliminate the cost-effectiveness associated with SCIT altogether. However, the data are clear that an economic “break-even” point remains for all NS tested. One explanation for why this curve only shifted slightly is that most of the expense in the SCIT with 50% reduction of NS arm lies in the specialty clinic visit charge, including the skin testing and not in the interventions, especially when considering a generic NS such as fluticasone or flunisolide. Unlike our analysis of repeat SCIT, which included presumed need for repeat skin testing, in this model, we are not positing the need for further re-evaluations in this population.
There are limitations to our analyses. It is possible that some of the nasal steroids mentioned may actually improve rhinitis symptoms better than others, although this has not been shown in one-on-one clinical trials. Alternatively, it has been suggested that SCIT may decrease the risk of future asthma and improve asthma symptoms and medication usage,46 which obviously would provide substantial benefits to SCIT not captured in this analysis. Our model only focused on the cost-effectiveness of SCIT versus NS for life and does not account for these improvements in quality of life.
In conclusion, our hypothetical model, based on computer simulation—and despite the use of stringent criteria in favor of NS—provides compelling evidence of the economic benefits of SCIT compared with NS, dependent on age at initiation of therapy and duration of use of the NS per annum. Even after accounting for only incomplete (50%) reduction in NS requirements or the requirement to reinitiate SCIT after loss of long-term tolerance, economic “break-even” points are evident. This analysis allows physicians to determine cost-effectiveness of continuing intranasal steroids for life or referring the patient for consideration for SCIT in a patient for whom NS therapy was previously effective. Decision-making models can assist the physician in accounting for these factors and customize patient counseling with regard to treatment options.
Footnotes
Funded by grants NIH RO1 AI1057483, NIH UO1: AI100799, NIH 5T32: AI007496-17, and the University of Virginia Children's Hospital Fellows Grant-in-Aid
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Alkire M. Driving out waste in health care. J Healthcare Financial Management Assoc 66:108–109, 2012. [PubMed] [Google Scholar]
- 2. Cauchi R, Hinkley K, Yondorf B. Great ideas for cutting costs: Six more strategies to manage the rising costs of health care. State Legislatures 38:28–31, 2012. [PubMed] [Google Scholar]
- 3. Goroll AH, Schoenbaum SC. Payment reform for primary care within the accountable care organization: A critical issue for health system reform. JAMA 308:577–578, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Holahan J, McMorrow S. Medicare and Medicaid spending trends and the deficit debate. N Engl J Med 367:393–395, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Roehrig C, Turner A, Hughes-Cromwick P, Miller G. When the cost curve bent: Pre-recession moderation in health care spending. N Engl J Med 367:590–593, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Blanchette CM, Dalal AA, Mapel D. Changes in COPD demographics and costs over 20 years. J Med Econ 15:1176–1182, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Gheorghian A, Schnitzler MA, Axelrod DA, et al. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation 94:241–249, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 367:299–308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollock R, Muduma G, Valentine W. Evaluating the cost-effectiveness of laparoscopic adjustable gastric banding versus standard medical management in obese patients with type 2 diabetes in the UK. Diabetes Obes Metab 15:121–129, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Cavanaugh TM, Buring S, Cluxton R. A pharmacoeconomics and formulary management collaborative project to teach decision analysis principles. Am J Pharmaceut Educ 76:115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diamantopoulos A, Sawyer L, Ogale S, Dejonckheere F. Validity of cost and utility results? Pharmacoeconomics 30:977, 2012. [DOI] [PubMed] [Google Scholar]
- 12. van Luijn JC. Is There a role for pharmacoeconomics in decision making? Pharmacoeconomics 30:979, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Akinbami LJ, Sullivan SD, Campbell JD, et al. Asthma outcomes: Healthcare utilization and costs. J Allergy Clin Immunol 129:S49–S64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerzeli S, Rognoni C, Quaglini S, et al. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig 32:253–265, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Hockenhull J, Elremeli M, Cherry MG, et al. A systematic review of the clinical effectiveness and cost-effectiveness of Pharmalgen(R) for the treatment of bee and wasp venom allergy. Health Technol Assess 16:III-IV, 1–110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ariano R, Berto P, Tracci D, et al. Pharmacoeconomics of allergen immunotherapy compared with symptomatic drug treatment in patients with allergic rhinitis and asthma. Allergy Asthma Proc 27:159–163, 2006. [PubMed] [Google Scholar]
- 17. Brüggenjürgen B, Reinhold T, Brehler R, et al. Cost-effectiveness of specific subcutaneous immunotherapy in patients with allergic rhinitis and allergic asthma. Ann Allergy Asthma Immunol 101:316–324, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Omnes LF, Bousquet J, Scheinmann P, et al. Pharmacoeconomic assessment of specific immunotherapy versus current symptomatic treatment for allergic rhinitis and asthma in France. Eur Ann Allergy Clin Immunol 39:148–156, 2007. [PubMed] [Google Scholar]
- 19. Petersen KD, Gyrd-Hansen D, Dahl R. Health-economic analyses of subcutaneous specific immunotherapy for grass pollen and mite allergy. Allergologia et Immunopathologia 33:296–302, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Pokladnikova J, Krcmova I, Vlcek J. Economic evaluation of sublingual vs subcutaneous allergen immunotherapy. Ann Allergy Asthma Immunol 100:482–489, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Lockey RF, Hankin CS. Health economics of allergen-specific immunotherapy in the United States. J Allergy Clin Immunol 127:39–43, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein JA. Pharmacoeconomic considerations for allergen immunotherapy. Clin Allergy Immunol 18:151–164, 2004. [PubMed] [Google Scholar]
- 23. Donahue JG, Greineder DK, Connor-Lacke L, et al. Utilization and cost of immunotherapy for allergic asthma and rhinitis. Ann Allergy Asthma Immunol 82:339–347, 1999. [DOI] [PubMed] [Google Scholar]
- 24. Hankin CS, Cox L, Lang D, et al. Allergen immunotherapy and health care cost benefits for children with allergic rhinitis: a large-scale, retrospective, matched cohort study. Ann Allergy Asthma Immunol 104:79–85, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Hankin CS, Cox L, Lang D, et al. Allergy immunotherapy among Medicaid-enrolled children with allergic rhinitis: patterns of care, resource use, and costs. J Allergy Clin Immunol 121:227–232, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Mattos JL, Woodard CR, Payne SC. Trends in common rhinologic illnesses: Analysis of US healthcare surveys 1995–2007. Int Forum Allergy Rhinol 1:3–12, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc 31:375–380, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: An updated practice parameter. J Allergy Clin Immunol 122:S1–S84, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Dykewicz MS, Kaiser HB, Nathan RA, et al. Fluticasone propionate aqueous nasal spray improves nasal symptoms of seasonal allergic rhinitis when used as needed (prn). Ann Allergy Asthma Immunol 91:44–48, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Jen A, Baroody F, de Tineo M, et al. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 105:732–738, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Kaszuba SM, Baroody FM, deTineo M, et al. Superiority of an intranasal corticosteroid compared with an oral antihistamine in the as-needed treatment of seasonal allergic rhinitis. Arch Intern Med 161:2581–2587, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007:CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol 125:131–138, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med 341:468–475, 1999. [DOI] [PubMed] [Google Scholar]
- 35. Mosbech H, Osterballe O. Does the effect of immunotherapy last after termination of treatment? Follow-up study in patients with grass pollen rhinitis. Allergy 43:523–529, 1988. [DOI] [PubMed] [Google Scholar]
- 36. Georgy MS, Saltoun CA. Chapter 3: Allergen immunotherapy: definition, indication, and reactions. Allergy Asthma Proc 33(Suppl 1):S9–S11, 2012. [DOI] [PubMed] [Google Scholar]
- 37. Larenas Linnemann DE. One hundred years of immunotherapy: Review of the first landmark studies. Allergy Asthma Proc 33:122–128, 2012. [DOI] [PubMed] [Google Scholar]
- 38. Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 116:377–383, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of allergic rhinitis: An analysis of randomized, prospective, single- or double-blind, placebo-controlled studies. Clin Therapeut 22:342–350, 2000. [DOI] [PubMed] [Google Scholar]
- 40. Schiller JS, Lucas JW, Ward BW, Peregory JA. Summary health statistics for US adults: National Health Survey, 2010. Vital Health Stat 10:1–207, 2012. [PubMed] [Google Scholar]
- 41. Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol 75:543–625, 1995. [PubMed] [Google Scholar]
- 42. Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy 57:306–312, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Hedlin G, Heilborn H, Lilja G, et al. Long-term follow-up of patients treated with a three-year course of cat or dog immunotherapy. J Allergy Clin Immunol 96:879–885, 1995. [DOI] [PubMed] [Google Scholar]
- 44. Jacobsen L, NũchelPetersen B, Wihl JA, et al. Immunotherapy with partially purified and standardized tree pollen extracts, IV: Results from long-term (6-year) follow-up. Allergy 52:914–920, 1997. [DOI] [PubMed] [Google Scholar]
- 45. Di Rienzo V, Marcucci F, Puccinelli P, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy 33:206–210, 2003. [DOI] [PubMed] [Google Scholar]
- 46. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: A practice parameter third update. J Allergy Clinical Immunol 127:S1–S55, 2011. [DOI] [PubMed] [Google Scholar]