Figure 6.
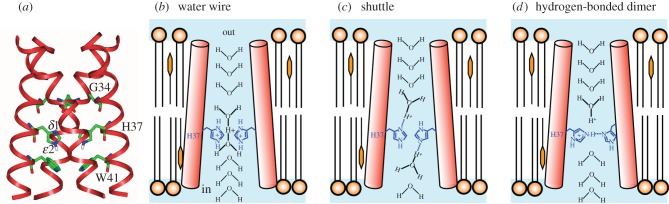
The well-studied M2 proton channel of influenza A virus is a homotetramer; (a) shows the high-resolution crystal structure with key residues labelled. Three proposals for the proton selective conduction are illustrated in (b–d), with only two of the four protomers shown for clarity. In the water wire model (b), the proton pathway is exclusively aqueous, with protonation of multiple His37 serving to open the pore by electrostatic repulsion. Proton selectivity in the shuttle model (c) is achieved by successive protonation and deprotonation of His37, with a ring flip completing each conduction event. In (d) protonated and unprotonated His37 form hydrogen-bonded dimers that are broken during H+ conduction. (Adapted from [139].)
