Abstract
While terrestrial insects can usually attach directly to a substrate, for aquatic insects the situation is more complicated owing to the presence of a biofilm on the primary substrates. This important fact has been neither the subject of investigation nor commonly taken into account in the interpretation of functional aspects of attachment in mobile freshwater animals. In this study, we investigate the impact of a biofilm on the attachment of living mayfly larvae. We performed in vivo attachment experiments in a flow channel using different substrates with defined surface roughness. Additionally, we measured friction forces directly generated by dissected tarsal claws on the same substrates. On substrates with smooth or slightly rough surfaces, which have little or no surface irregularities large enough for the claws to grasp, the presence of a biofilm significantly increases the friction force of claws. Consequently, larvae can endure higher flow velocities on these smooth substrates. The opposite effect takes place on rough substrates, where the friction force of claws decreases in the presence of a biofilm. Consequently, a biofilm is a critical ecological structure for these larvae, and other aquatic organisms, not only as a food source but also as a factor influencing attachment ability.
Keywords: periphyton, surface texture, claw, roughness, friction, Epeorus
1. Introduction
When terrestrial insects attach to a substrate, they do so directly. However, in aquatic environments, there is a biofilm coating primary substrates, so contact is no longer direct. After just 2 h of exposure in an aquatic environment, organic material, bacteria and fungi form a primary biofilm on the surface of the substrate [1]. Subsequently, autotroph microorganisms and algae attach. The microorganisms secrete extracellular polymer substances (EPSs), which embed algae, bacteria, fungi and detritus particles in an organic sublayer [2]. Biofilms are complex highly hydrated structures, which show population heterogeneity in space and time [3].
Considering interactions of benthic animals with the biofilm, the relevance of the biofilm as a food source for grazers has been intensively investigated [4–10]. By contrast, its influence on animal attachment has not garnered much attention to date. While biofilms can vary greatly in composition and thickness [2], they are usually softer than the primary substrate, and change surface structure and chemistry. Such factors, substrate roughness, stiffness, wettability, chemistry and temperature, can have considerable effects on the adhesive strength of benthic animals [11].
Most of the literature dealing with the functional aspects of attachment of freshwater macroinvertebrates [12–19] does not investigate the possible influence of the biofilm surface. In some sessile freshwater animals, for example Dreissena polymorpha, the attachment ability is not influenced by a biofilm [20]. This is owing to the fact that Dreissena mussels replace the biofilm with their byssal threads and make direct contact with the substrate [21]. Nevertheless, in the presence of a biofilm, a higher number of individuals was observed during the first step of colonization [22]. By contrast, simuliid larvae show no difference in abundance during first colonization, but it has been assumed that a biofilm has an effect on long-term settlement and attachment [23]. For many marine sessile animals, such as mussels, bryozoans, coelenterates or polychaetes, a biofilm can be either a settlement cue or inhibitor [23–29].
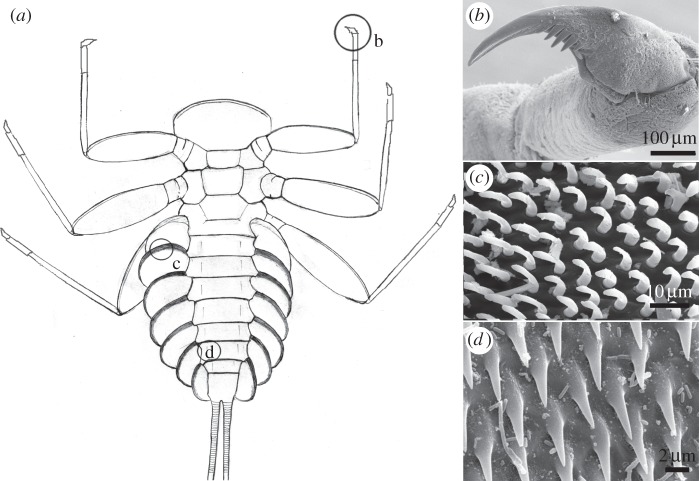
Sessile animals, however, are not alone in having to deal with a biofilm and, to our knowledge, the impact of biofilms on mobile insect larval attachment remains unexplored. Our recent investigations indicate that the presence of a biofilm considerably influences the ability of some aquatic insect larvae to attach to the substrate surface. For example, the reported ability of the running water mayfly Epeorus to attach to smooth surfaces [30,31] was no longer observed on smooth sterile substrates [32]. Besides a single tarsal claw, these mayfly larvae are equipped with additional attachment devices, for example friction pads (figure 1), which contribute to the general attachment force of the insect [31,33]. Surface roughness varies greatly in natural aquatic substrates and has a significant impact on the forces exerted by different attachment devices in E. assimils as well as in other insects [31,32,34]. However, the impact of a biofilm, especially in conjunction with surface roughness, has not been investigated.
Figure 1.
Attachment devices of E. assimilis larvae: (a) ventral view, (b) claw of the foreleg, (c) setae of the pads on the ventral side of the gill lamellae and (d) areas with spiky acanthae on the lateral parts of the abdominal sternits. (Adapted from [31].)
The aim of this study is to understand the role of the biofilm in the attachment of mobile insect larvae, using the mayfly Epeorus assimilis as our model organism. We investigated the following questions. (i) Does the biofilm influence the ability of live E. assimilis larvae to attach to the substrate? (ii) Does the biofilm influence the friction forces generated by the tarsal claws? (iii) Is there any combined influence of biofilm and the surface roughness of the primary substrate on the mayfly's attachment ability?
2. Material and methods
2.1. Animals
The last instars of E. assimilis Eaton 1885 larvae were collected from streams located in the Taunus Forest close to Weilrod, Germany. The live larvae were packed on ice and were transported to the laboratory flume at the Federal Institute of Hydrology in Koblenz, Germany. The maximum transport time was approximately 1.5 h. A number of live specimens were fixed in 70% ethanol and were used to measure the friction force generated by the claws.
2.2. Substrates
In contrast to heterogeneous natural substrates, for example stone, artificial substrates can be selected according to which of their specific properties (stiffness, roughness, etc.) are of interest. Replication techniques allow the preparation of substrates with relatively homogeneous chemical composition but different, defined surface roughness.
For the experiments, we prepared substrates with four different surface roughnesses, chosen in accordance with former attachment studies focusing on the roughness impact without biofilm formation [31,32]. We used a two-step moulding process, using dental wax (President Light Body; Coltène Whaledent, Langenau, Germany) and epoxy resin-containing hardener (Epoxidharz L and Härter S; Conrad Electronics, Hirschau, Germany) in accordance with Koch et al. [35], to create replicate substrates. Glass (S1) replicas were used as a smooth reference. Moreover, replicas (size: 15 × 20 cm) were made of three different surface roughnesses: special polishing paper with a nominal asperity size of 1 µm (S2) and 12 µm (S3) (523 and 545; Buehler, Lake Bluff, IL, USA) and normal polishing paper 400 P (S4) (Tyage; Wolfcraft, Kempenich, Germany). These epoxy replicas were used both with and without a biofilm. To cultivate a biofilm of sufficient thickness, replicas of all substrate types were exposed in the laboratory flume for three weeks before the experiments started. During the daytime, substrates were exposed to artificial light. After the experiments, some biofilm samples were frozen and stored at −70°C for later examination (confocal laser scanning microscopy (CLSM), mechanical properties).
2.3. Confocal laser scanning microscopy
Thawed substrate samples were carefully transferred to water. In the case of the roughest substrate without a biofilm, air remained adhered to the surface, which was removed by briefly exposing the substrate to a vacuum and adding a few droplets of Triton X-100 (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) to the water. Later, the mixture of water and Triton X-100 was replaced by pure water. The surface topography of all substrates was analysed by applying the confocal laser scanning microscope Zeiss LSM 700 (Carl Zeiss Microscopy GmbH, Jena, Germany) equipped with an upright microscope (Zeiss Axio Imager.M1m). For the visualization, a 25× objective (Zeiss Plan-Apochromat, multi-immersion (oil, glycerine and water) objective, numerical aperture = 0.8; directly immersed in the water above the substrates) was used. The substrates were exposed to light from a stable solid-state laser with a wavelength of 405 nm (5 mW at the fibre end, laser power = 1.5%), and the laser light reflected at the surface of the substrates was detected. Detector gain was manually adjusted prior to image stack collection in a way resulting in maximum signal intensity while simultaneously preventing oversaturation. Digital gain and digital offset were set to 1 and 0, respectively. We used the CLSM software Zeiss Efficient Navigation (ZEN) to automatically determine the optimal image size (limited by the CLSM system to a maximum of 2048 × 2048 pixels) necessary according to the Nyquist–Shannon sampling theorem. A pinhole size of 0.33 Airy units was applied. For each image stack, overlapping optical slices were visualized for the entire z-range of the surface. The distance between two consecutive focal planes was chosen in a way that the sampling rate was two times larger than necessary according to the Nyquist–Shannon sampling theorem. All image stacks were collected with a line average of 2. Scan rates resulting in a combination of both a very good signal-to-noise ratio and a reasonable collecting time were selected. ZEN software was used to create colour-coded height maps and to calculate the roughness parameters from the image stack data. For this purpose, no filter was applied, and the surfaces were fitted to a plane.
2.4. Mechanical properties of the biofilm
For testing, thawed biofilm samples on glass slides were fixed on the bottom of a Petri dish filled with tap water. Indentation measurements were performed under water using force tester Basalt-2 [36], a home-made microindentation set-up. It consists of a sample stage located on a six-axial nanopositioning system (Hexapod F206; PI, Karlsruhe, Germany), a flat glass spring (spring constant = 287 N m−1) and a mini-interferometer SP-120 (SIOS Meßtechnik GmbH, Jena, Germany), fixed on a piezo stage (P611 nanocube stage; PI, Karlsruhe, Germany). An indenting sapphire sphere (radius = 1.5 mm, Goodfellow, UK) and a mirror were glued to the spring. The mirror displacement proportional to the force applied to the spring was monitored using an interferometer. The set-up was installed on a vibration isolation table (TS-150; Table Stable Ltd, Ammerbuch, Germany). The Hexapod nanopositioning system was controlled by means of the manufacturer's software. Load–displacement curves (figure 2) were acquired using a custom-made program written in LabVIEW (National Instruments, Austin, TX, USA).
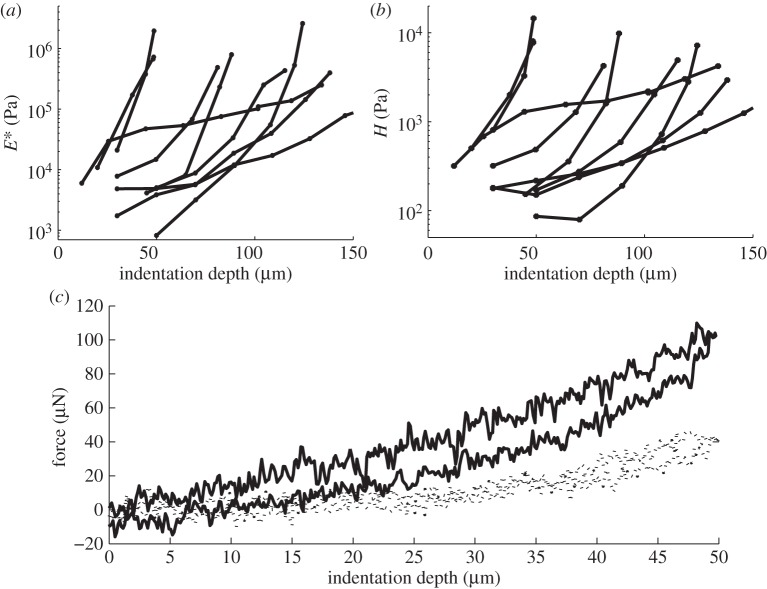
Figure 2.
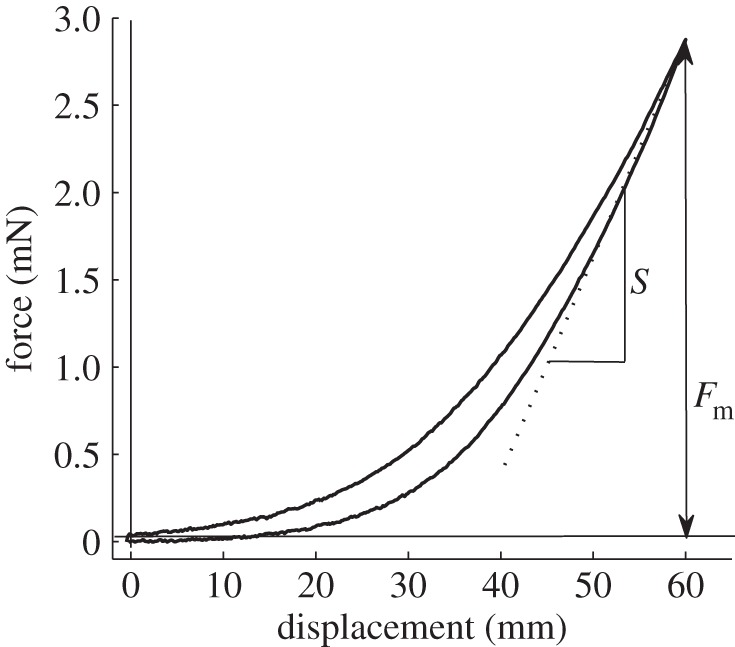
Example of a force–distance curve obtained in biofilm indentation experiments. Fm is the maximum applied force measured at the maximum indentation depth and S is the slope of the force–distance curve at Fm.
As there was no adhesion observed in the biofilm indentation tests, the Oliver–Pharr model [37] was used to determine Young's modulus (Er) and hardness (H). The following equations were derived from the model:
where Fm is the maximum applied force (figure 2), δm is the maximum indentation depth, hc is the vertical distance along which contact is made (the contact depth), R is the radius of the indentation sphere and S is the slope of the force–distance curve at δm. The experimental data were analysed in Matlab (The MathWorks Inc.).
2.5. Estimation of insect attachment ability in a laboratory flume
Attachment experiments were carried out in an artificial stream flume (for details, see [33]). Substrates S1–S4 were placed in the flume and arranged so that each replicate experienced comparable flow conditions. For each experimental run, E. assimilis larvae were placed on a single substrate replica, when the flow velocity was zero. Then, the rotational speed of the paddle wheel was gradually increased until the animal left the substrate or the maximum speed of the flume (more than 40 cm s−1 bottom flow velocity) was reached. For each type of substrate, the experiment was repeated using 10 or 11 animals.
Experiments were recorded using a videoscope Iplex II camera (Olympus, Hamburg, Germany). Selected video sequences were analysed frame by frame using SIS Bildanalyse software EIS (Olympus, Münster, Germany). For calibration of distances and dimensions, a ruler was placed in the background during video recording. Using this scale, the bottom flow velocity was determined from the shutter speed of the video camera and from the length of the lines traced by single particles in single video frames.
2.6. Friction measurements at claw tips of isolated insect legs
The right foreleg was dissected from larvae, fixed in 70% ethanol and cut between the trochanter and femur. A special clamp was used to clip single legs to the force sensor, oriented as naturally as possible: with the tibia parallel to the substrate and the claw in contact with the substrate. The force transducer was mounted on a motorized micromanipulator (MS314, M/W; Märzhäuser, Wetzlar, Germany), which was moved parallel to the substrate with a constant velocity of 100 µm s−1. During movement, the friction force was monitored by load cell force transducers (10 and 25 g capacity; Biopac Systems Ltd, Santa Barbara, CA, USA). Normal forces applied by the claw to the substrate were measured and averaged 656 ± 311 µN for the entire series of experiments. Friction measurements were performed on all substrates with and without a biofilm.
3. Results
3.1. Surface properties of the substrates
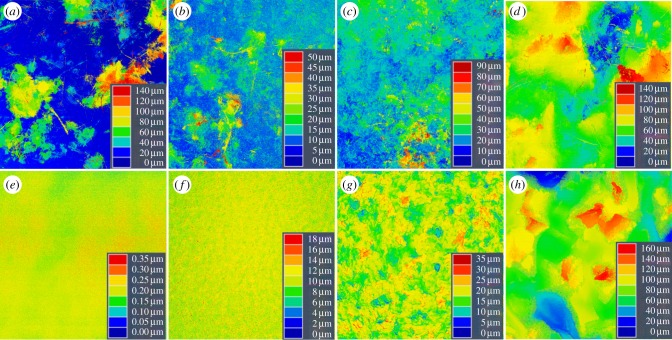
The biofilm creates a secondary surface, on top of the primary substrate, which modifies the primary structure (figure 3). The most dramatic differences were observed on the smooth substrate (S1), where the height of the surface asperities increased considerably with the addition of a biofilm, from less than 1 µm to more than 100 µm. On the substrates with a slight (S2) and medium (S3) roughness, the range of variation in height was more than two and a half times higher after the growth of a biofilm. By contrast, on the rough substrate (S4), the range of variation in height with biofilm was almost equal to the range of variation without. The described differences are reflected in the calculated roughness parameters (table 1). For example, the maximum roughness depth (Rmax) shows a significant increase in surface roughness in the presence of a biofilm for the substrates S1–S3 (t-test: S1: p < 0.005, d.f. = 3, t = 18.22; S2: p = 0.016, d.f. = 3, t = 4.97; S3: p < 0.049, d.f. = 3, t = 3.22). By contrast, no significant difference was observed on substrate S4 (t-test: p = 0.169, d.f. = 3, t = −1.80).
Figure 3.
CLSM height maps showing the surface structure of the substrates: substrates with (a–d) and without (e–h) a layer of biofilm. On the smoother substrates, the presence of the biofilm layer resulted in an increased surface roughness compared with that of the primary surface (S1: a,e; S2: b,f; S3: c,g). On the substrates with the coarse initial surface roughness, the roughness remained almost the same after covering the surface with the biofilm (S4: d,h). S1, 100 × 100 µm; S2–S4, 512 × 512 µm. (Online version in colour.)
Table 1.
Roughness parameters of the investigated substrates with and without biofilm, mean ± s.d. Rmax, maximum roughness depth; Ra, roughness average.
|
Rmax (µm) |
Ra (µm) |
|||
|---|---|---|---|---|
| without biofilm | with biofilm | without biofilm | with biofilm | |
| S1 | 0.50 ± 0.16 | 177.90 ± 19.47 | 0.036 ± 0.01 | 32.42 ± 20.06 |
| S2 | 21.32 ± 2.14 | 66.28 ± 17.99 | 1.33 ± 0.11 | 5.13 ± 2.93 |
| S3 | 36.52 ± 1.74 | 67.47 ± 19.16 | 3.19 ± 0.11 | 5.09 ± 1.65 |
| S4 | 196.05 ± 44.01 | 153.26 ± 17.85 | 21.99 ± 2.6 | 18.17 ± 0.94 |
The biofilm that developed on the test substrates was around 200 µm thick. The mechanical properties of the biofilm were considerably different from those of the primary substrate. On average, Young's modulus of the biofilm was 188 ± 112 kPa (mean ± s.e.m.). However, the material was strongly non-homogeneous, and Young's modulus varied from 0.812 to 2584 kPa (figure 4a). Hardness values were non-homogeneous as well and ranged from 0.079 to 14.55 kPa, with a mean value of 2.06 ± 0.41 kPa (figure 4b). Both Young's modulus and hardness values increased with indentation depth. One reason for this is a gradient of mechanical properties in a biofilm; nevertheless, the artefact caused by the stiff support cannot be excluded. Algae cells attaching to the support provide different mechanical properties from the gel-like EPS of the biofilm. Overall, the biofilm deformed elasto-plastically, and biofilm adhesion was not detectable. After load application, a pit appeared on the surface of the biofilm, which was seen as a successive shift of indentation curves (figure 4c).
Figure 4.
Young's modulus (a) and hardness (b) depend on indentation depth at different locations (different curves). Residual plastic deformation (c) is demonstrated as a shift of an indentation curve (dotted curve) after application of 2.13 kPa of average stress to the biofilm. Indentation curve, while applying stress of 2.13 kPa, is plotted as a solid line.
3.2. The influence of the biofilm on the attachment of mobile mayfly larvae
On most substrates, attachment ability of live E. assimilis larvae changed in the presence of biofilm (figure 5; see electronic supplementary material). On substrates which provided no or marginal surface irregularities to grasp (S1–S3), the larvae were able to attach more strongly to substrates covered by biofilm than to bare substrates. The larvae were also able to stay on these substrates at higher flow velocities than on the bare substrates (Wilcoxon–Mann–Whitney: S1: W = 62.5, p < 0.005; S2: W = 63.0, p < 0.005; S3: W = 62.5, p < 0.005). By contrast, there were no differences found in the attachment ability on rough substrates (S4) with or without biofilm (Fisher's exact test: p = 1), and larvae stayed attached up to a bottom flow velocity of more than 40 cm s−1 on both. However, as this was the maximum flow velocity of the flume, we cannot rule out differences in attachment ability for the S4 substrate with and without biofilm at higher flow velocities. Video analysis showed that larvae primarily move a single leg, sometimes two, while moving forwards. In some cases, we observed that claws slowly slid backwards and the larvae had to re-adjust to maintain their position.
Figure 5.
Attachment ability of E. assimilis larvae on substrates with different surface roughness. The same experiment was performed for the same substrates with and without biofilm. The maximal flow velocity at which the larvae remained on each substrate was determined. In the presence of the biofilm, more larvae stayed on substrates S1–S3 at higher flow velocities. Almost all larvae were able to withstand the maximum flow velocity with and without biofilm on the substrate S4. S1–S4, substrates of the types 1–4, respectively.
3.3. Claw-generated attachment force on the substrates with and without biofilm
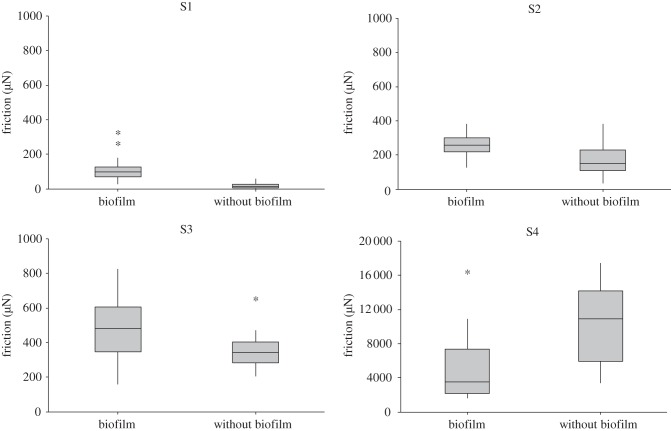
We measured the forces generated by the tarsal claws of dissected legs parallel to the substrate to isolate the influence of the biofilm on the claws versus the influence on the other attachment structures of Epeorus larvae. The claw-generated attachment force increased significantly on smooth and slightly rough substrates in the presence of the biofilm (S1: Mann–Whitney, W = 768, N = 44, p < 0.001; S2: two-sample t-test, T = 4.88; d.f. = 51, p < 0.001; S3: Mann–Whitney, W = 721, N = 46, p < 0.05). The opposite outcome was observed on the rough substrate (S4; figure 6; see electronic supplementary material), here the attachment force of the claws was lower for the substrate covered with the biofilm (S4: two-sample t-test, T = 3.01; d.f. = 43; p < 0.005).
Figure 6.
Attachment forces of claws measured on the substrates with and without biofilm while pulling laterally. On the smooth substrate (S1) and on the substrates with the slight and medium surface roughness (S2 and S3), forces generated by the claw were significantly higher in the presence of the biofilm. By contrast, on the coarse roughness (S4), the forces were lower in the presence of the biofilm. Graphs are box plots with median, upper and lower quartiles, interquartile range and outliner values. S1–S4, substrate types 1–4, respectively.
4. Discussion
Anyone who has slipped on wet stones in a stream is familiar with the effects of biofilm cover. One might expect it to be more difficult for aquatic insect larvae to attach to a substrate covered with this biofilm. This study, however, demonstrates that on substrates with a smooth or slightly rough surface structure quite the opposite happens.
Depending on tarsal claw tip size, there is a species-specific minimum roughness past which claws cannot find sufficient surface irregularities to grasp onto primary substrates [32,38]. For these kinds of substrates (in this study: S1, S2 and S3), friction measurements demonstrate that the presence of biofilm leads to a significant increase in forces between the excised claws and the substrate. One possible explanation for this is that the biofilm, or the attached organisms that make the biofilm, increases the number and the amplitude of surface irregularities that tarsal claws can grasp. By contrast, on very rough substrates (in this study: S4), the particles and organisms of the biofilm can accumulate in the valleys of the substrate and decrease the overall change in amplitude. Nevertheless, even the roughness of the biofilm-covered substrate remains sufficient to generate high attachment forces. On these very rough surfaces, interlocking of the tarsal claws with surface irregularities plays an important role in attachment.
Moreover, the EPS secreted by organisms in the biofilm changes the material properties of the substrate that the insect larva is interacting with. Our measured Young's modulus (about 200 kPa) is in the range of values given in the literature for algae (100–500 kPa), bacteria (50–2000 kPa) and for the soft parts of a drinking water biofilm (less than 600 kPa) [39,40]. These low values for Young's modulus indicate the low stress needed to stretch or compress soft biofilm material. The increase in the Young's modulus close to the primary substrate surface is related to the primary substrate's effect on the indentation measurements [41], but also a gradient in the biofilm might contribute to this increase. Algal cells attaching to the substrate are expected to have higher values of elasticity modulus, like the gel-like EPS. We found that, unfortunately, there is no simple method for reconstructing a depth profile of either the elasticity modulus or hardness for such heterogeneous systems. Using the Oliver–Pharr method, it was found that the overestimation of both values, caused by the finite biofilm thickness, is about 30% at an indentation depth of around 0.75 [41]. This depth-dependent increase is negligible compared with the increase of that observed in the experiment for both hardness and elasticity. Therefore, the biofilm has an extremely strong depth gradient for both Young's modulus and hardness. Nevertheless, the average stress applied by the tip of a claw (according to the Hertz theory for 1 mN applied force and 3 µm radius of the claw's tip) is higher than the observed maximum biofilm hardness, even when measured at its deepest layers and close to the substrate (H ∼ 15 kPa) at Young's modulus higher than 0.7 kPa. Moreover, the hardness of the claws' chitin is orders of magnitude higher than the hardness of the biofilm. While the mean value of biofilm hardness in this study was 2.06 ± 0.41 kPa, in the literature there are hardness values reported for hydrated Schistocerca claws of 15 VHN (Vickers hardness numbers; 15 VHN is around 15 MPa) [42]. The tarsal claws used in this study are longer (length between the claw tip and the ventral–proximal edge of the claw is around 330 µm [32]) than the thickness of the biofilm (around 200 µm). Thus, the claws can freely pierce the gel-like biofilm and interlock with substrate irregularities caused by particles and algal cells attaching to the bare substrate (figure 7). Additionally, the biofilm surrounding the claw may increase resistance while claws are pulling parallel to the substrate owing to its high viscosity. On the other hand, the EPS of the biofilm between the claw tip and the substrate may also act as a lubricant and reduce friction.
Figure 7.
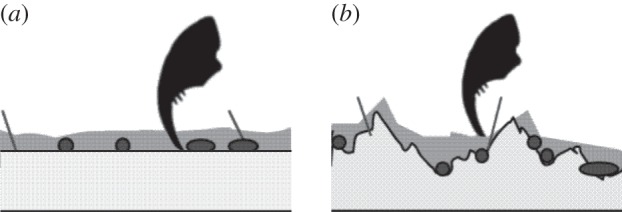
Diagram of the hypothetical interaction between claws and substrates covered with the biofilm. (a) The organisms of the biofilm and their EPSs increase the surface roughness of smooth substrates. (b) On rough substrates, surface roughness is not further increased. If the organisms of the biofilm preferably attach to the substrate valleys, the surface roughness might even be reduced. On smooth and rough substrates, claws might penetrate the EPSs.
It is important to remember that the composition and thickness of biofilms can strongly vary [2]. The thickness of the biofilm in our study is within the range of other river microbial biofilms, both those generated in a laboratory and those measured in the field [2,3,43]. Because of this, we can assume comparable thickness under most natural conditions. However, during winter, when there are fewer grazing invertebrates present, the thickness of the biofilm can increase up to a few mm [3]. This can be a considerably different situation for the remaining benthic invertebrates, as their claws may not be able to reach the primary substrate underneath. While biofilm structure and properties, such as elasticity, hardness and plasticity, may vary in different biofilms, increased surface roughness on smooth substrates and a layer of EPS can be expected for most types of biofilm. Therefore, the general trend in attachment forces for small-sized benthic animals on biofilm-covered substrates (of a comparable thickness) can be expected to be approximately the same. Thus, live larvae should be able to endure higher flow velocities on smooth substrates covered with a biofilm than without. This could also mean that drifting larvae may be better able to reattach, because reattachment begins with the tarsal claws [32,44]. On rough substrates, the attachment force of the claws decreased in the presence of biofilm, which may be the result of the lubricating effect of the biofilm EPS. However, this might not be biologically relevant for the live larvae, because their attachment force is about 8–40 times higher on the bare rough primary surfaces (S4) than on the smoother substrates with biofilm (S1–S3). In our experiments, live larvae showed no difference in their ability to attach to rough substrates with or without biofilm. Nevertheless, we cannot exclude the possibility that there might be differences at higher flow velocities.
In the resting position, all six legs of E. assimilis larvae are in contact with the substrate, and when they move, only one or two legs move at a time. Assuming that a minimum of four claws are grasping the substrate, we can estimate an overall larval attachment force of approximately 0.4 mN on S1, of approximately 1.2 mN on S2, of approximately 2 mN on S3 and of approximately 16 mN on S4. Applying Cw-values taken from the literature [45], we can estimate drag forces acting on the larvae parallel to the substrate according to the formula Fd = 1/2 CdρSv² (Re numbers were in the same range; Fd, drag forces; Cd, drag coefficient; ρ, water density; S, area in frontal projection; and v, flow velocity). At very high flow velocities of 2 m s−1, which are typical for torrential streams [46], calculated drag forces reach approximately 20 mN, which is higher than our calculated attachment forces. At flow velocities up to 20 cm s−1, we calculated drag forces of no more than approximately 0.2 mN, which is less than the attachment force generated by four claws on all substrates tested. At higher flow velocities, the drag forces are higher than the calculated attachment forces for S1 (v = 0.3 ms−1, Fd = 0.5 mN; v = 0.4 m s−1, Fd = 0.9 mN) or S2 (v = 0.5 m s−1, Fd = 1.3 mN). Despite this, 30% (S2, v > 0.4 m s−1) to 50% (S1, v > 0.2 m s−1) of the larvae were able to remain attached in these higher flow velocities. Possibly this disconnect is owing to variation in actual attachment forces corresponding to varying biofilm thickness, but other attachment mechanisms cannot be ruled out.
Our experiments were performed with the mayfly larva E. assimilis, and claws are not the only mechanism for attachment. These larvae also have special setose attachment pads on the ventral side of their gill lamellae, which are in close contact with the substrate when the larva rests in stronger currents. These attachment pads increase friction on sterile smooth substrates and might increase friction on the biofilm as well [31]. We hypothesize that both components together, the increased attachment force of the claws and the friction generated by attachment pads, can increase the friction force up to a value that enables the animals to inhabit smooth stones with a biofilm cover even while being exposed to higher flow forces.
Attachment is further complicated because larvae also experience lift forces acting perpendicular to the substrate, in addition to drag forces [47,48]. These lift forces can be even higher than the drag forces, but can vary greatly under similar flow velocities [45]. These lift forces might be the reason that live larvae were not able to remain on the substrates S2 and S3 without a biofilm, even if attachment forces of isolated claws (S2: 0.6 mN, S3: 1.4 mN for four claws) were higher than the calculated drag forces. The claws might not be able to grasp the substrate in a way that would withstand these tangential forces. On the other hand, the biofilm surrounding the claws can increase resistance, not only to drag but also to lift, owing to its viscosity, like placing the feet in honey.
We conclude that biofilm has a significant influence on the ability of E. assimilis larvae and other aquatic insects to attach to the substrate. Biofilm significantly increases friction forces on smooth and slightly rough substrates, where tarsal claws would not otherwise be able to find surface irregularities to grasp. Thus, larvae are able to stay on the same substrate, covered in biofilm, in higher flow velocities. Consequently, biofilm is of ecological relevance for the insect larvae not only as a food source but also as an important factor in their ability to remain attached in stream habitats. Our results show the importance of taking into account the effects of biofilm when dealing with questions of aquatic attachment. Moreover, these findings can inform future work in other fields, such as antifouling research or the development of artificial attachment devices for underwater purposes.
Acknowledgements
We thank Carola Winkelmann and Bernd Mockenhaupt (both Federal Institute of Hydrology, Koblenz, Germany) for support with the laboratory flume and with the cultivation of the biofilm. Stephanie Crofts (University of Washington) is greatly acknowledged for improving the language of the manuscript.
Funding statement
The German Research Foundation (DFG GO 10-1) supported parts of this work.
References
- 1.Korte VL, Blinn DW. 1983. Diatom colonization on artificial substrata in pool and riffle zones studied by light and scanning electron microscopy . J. Phycol. 19, 332–341. ( 10.1111/j.0022-3646.1983.00332.x) [DOI] [Google Scholar]
- 2.Eitner A. 2004. Struktur und Entwicklung benthischer Biofilme in Fließgewässern—Messung und Simulation. PhD thesis, Technische Universität Berlin, Berlin, Germany. [Google Scholar]
- 3.Neu TR, Lawrence JR. 1997. Development and structure of microbial biofilms in river water studied by confocal laser scanning microscopy. FEMS Microbiol. Ecol. 24, 11–25. ( 10.1111/j.1574-6941.1997.tb00419.x) [DOI] [Google Scholar]
- 4.Schwenk W, Schwoerbel J. 1973. Untersuchungen zur Ernährungsbiologie und Lebensweise der Flußmützenschnecke Ancylus fluviatilis (O.F. Müller 1774; Gastropoda Basommatophora). Arch. Hydrobiol. Suppl. 42, 190–231. [Google Scholar]
- 5.Swamikannu X, Kyle D, Hoagland KD. 1989. Effects of snail grazing on the diversity and structure of a periphyton community in a eutrophic pond. Can. J. Fish. Aquat. Sci. 46, 1698–1704. ( 10.1139/f89-215) [DOI] [Google Scholar]
- 6.Arens W. 1989. Comparative functional morphology of the mouthparts of stream animals feeding on epilithic algae. Arch. Hydrobiol. Suppl. 83, 253–254. [Google Scholar]
- 7.Arens W. 1994. Striking convergence in the mouthpart evolution of stream-living algae grazers . J. Zool. Syst. Evol. Res. 32, 319–343. ( 10.1111/j.1439-0469.1994.tb00490.x) [DOI] [Google Scholar]
- 8.Becker G. 1990. Comparison of the dietary composition of epilithic trichopteran species in a first-order stream. Arch. Hydrobiol. 120, 123–140. [Google Scholar]
- 9.Wellnitz TA, Ward JV. 1998. Does light intensity modify the effect mayfly grazers have on periphyton? Freshw. Biol. 39, 135–149. ( 10.1046/j.1365-2427.1998.00270.x) [DOI] [Google Scholar]
- 10.Liess A, Hillebrand H. 2004. Invited review: direct and indirect effects in herbivore–periphyton interactions. Arch. Hydrobiol. 159, 433–453. ( 10.1127/0003-9136/2004/0159-0433) [DOI] [Google Scholar]
- 11.Scherge M, Gorb SN. 2001. Biological micro- and nanotribology. Berlin, Germany: Springer. [Google Scholar]
- 12.Steinmann P. 1907. Die Tierwelt der Gebirgsbäche—eine faunistische Studie. Ann. Biol. Lacustre 2, 30–150. [Google Scholar]
- 13.Hora SL. 1927. The mechanism of the so-called ‘posterior sucker’ of Simulium larvae. Nature 119, 599–600. ( 10.1038/119599b0) [DOI] [Google Scholar]
- 14.Hora SL. 1930. Ecology, bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Phil. Trans. R. Soc. Lond. B 218, 171–282. ( 10.1098/rstb.1930.0005) [DOI] [Google Scholar]
- 15.Dodds GS, Hisaw FL. 1924. Ecological studies of aquatic insects. I. Adaptations of mayfly nymphs to swift streams. Ecology 5, 137–148. ( 10.2307/1929011) [DOI] [Google Scholar]
- 16.Dodds GS, Hisaw FL. 1925. Ecological studies of aquatic insects. III. Adaptations of caddies fly larvae to swift streams. Ecology 6, 123–137. ( 10.2307/1929367) [DOI] [Google Scholar]
- 17.Stuart M. 1958. The efficiency of adaptive structures in the nymph of Rhithrogena semicolorata (Curtis, Ephemeroptera). J. Exp. Biol. 35, 27–38. [Google Scholar]
- 18.Hynes HBN. 1970. The ecology of running waters. Liverpool, UK: Liverpool University Press. [Google Scholar]
- 19.Frutiger A. 2002. The function of the sucker of larval net-winged midges (Diptera: Blephariceridae). Freshw. Biol. 47, 293–302. ( 10.1046/j.1365-2427.2002.00814.x) [DOI] [Google Scholar]
- 20.Crisp DJ, Walker G, Young GA, Yule AB. 1985. Adhesion and substrate choice in mussels and barnacles. J. Colloid Interface Sci. 104, 40–50. ( 10.1016/0021-9797(85)90007-4) [DOI] [Google Scholar]
- 21.Baier RE, Gucinski H, Olivieri MP, Meyer AE. 1992. Rapid underwater bond formation and adhesion of zebra mussel attachment disc protein. In Proc. 6th Int. Symp. Structure of Adhesive Bondings, Morristown, NJ, 4–7 May 1992, pp. 1–9. Arlington, VA: American Defense Preparedness Association. [Google Scholar]
- 22.Wainmann BC, Hincks SS, Kaushik NK, Mackie GI. 1996. Biofilm and substrate preference in the dreissenid larvae of Lake Erie. Can. J. Fish. Aquat. Sci. 53, 134–140. ( 10.1139/f95-213) [DOI] [Google Scholar]
- 23.Kiel E. 1996. Effects of Aufwuchs on colonization by simuliis (Smuliidae, Diptera) . Int. Rev. Ges. Hydrobiol. 81, 565–576. ( 10.1002/iroh.19960810410) [DOI] [Google Scholar]
- 24.Kitamura H, Hirayama K. 1987. Effect of cultured diatom films on the settlement of larvae of a bryozoan Bugula neritina) . Nippon Suisan Gakkaishi 53, 1383–1385. ( 10.2331/suisan.53.1383) [DOI] [Google Scholar]
- 25.Kitamura H, Hirayama K. 1987. Effect of primary films on the settlement of larvae of a bryozoan Bugula neritina. Nippon Suisan Gakkaishi 53, 1377–1381. ( 10.2331/suisan.53.1377) [DOI] [Google Scholar]
- 26.Maki JS, Rittschof D, Samuelsson MO, Szewzyk U, Yule AB, Kjelleberg S, Costlow JD, Mitchell R. 1990. Effects of marine bacteria and their exopolymers on the attachment of barnacle cypris larvae. Bull. Mar. Sci. 46, 499–511. [Google Scholar]
- 27.Hadfield MG, Paul VJ. 2001. Natural chemical cues for settlement and metamorphosis of marine-invertebrate larvae. In Marine chemical ecology (eds McClintock JB, Baker JB.), pp. 431–460. Boca Raton, FL: CRC Press. [Google Scholar]
- 28.Dahms HU, Dobretsov S, Qian PY. 2004. The effect of bacterial and diatom biofilms on the settlement of the bryozoans Bulgula neritina. J. Exp. Mar. Biol. Ecol. 313, 91–209. ( 10.1016/j.jembe.2004.08.005) [DOI] [Google Scholar]
- 29.Bao WY, Satuito CG, Yang JL, Kitamura H. 2007. Larval settlement and metamorphosis of the mussel Mytilus galloprovinicilis in response to biofilms. Mar. Biol. 50, 565–574. [Google Scholar]
- 30.Ambühl H. 1959. Die Bedeutung der Strömung als ökologischer Faktor. Schweiz. Z. Hydrol. 21, 133–264. [Google Scholar]
- 31.Ditsche-Kuru P, Koop JHE, Gorb SN. 2010. Underwater attachment in current: the role of setose attachment structures on the gills of the mayfly larvae Epeorus assimilis (Ephemeroptera, Heptageniidae) . J. Exp. Biol. 213, 1950–1959. ( 10.1242/jeb.037218) [DOI] [PubMed] [Google Scholar]
- 32.Ditsche-Kuru P, Barthlott W, Koop JHE. 2012. At which surface roughness do claws cling? Investigations with larvae of the running water mayfly larvae Epeorus assimilis (Heptageniidae, Ephemeroptera). Zoology 115, 379–388. ( 10.1016/j.zool.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 33.Ditsche-Kuru P, Koop JHE. 2009. New insights into a life in current: do the gill lamellae of Epeorus assimilis and Iron alpicola larvae (Ephemeroptera: Heptageniidae) function as a sucker or as friction pads? Aquat. Insect 31, 495–506. ( 10.1080/01650420903106731) [DOI] [Google Scholar]
- 34.Gorb S. 2001. Attachment devices of insect cuticle. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 35.Koch K, Schulte AJ, Fischer A, Gorb SN, Barthlott W. 2008. A fast, precise and low-cost replication technique for nano- and high-aspect ratio structures for biological and artificial surfaces. Bioinspir. Biomim. 3, 1–10. ( 10.1088/1748-3182/3/4/046002) [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich H, et al. 2011. Calcite reinforced silica–silica joints in the biocomposite skeleton of deep-sea glass sponges. Adv. Funct. Mat. 21, 3473–3481. ( 10.1002/adfm.201100749) [DOI] [Google Scholar]
- 37.Oliver WC, Pharr GM. 1992. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583. ( 10.1557/JMR.1992.1564) [DOI] [Google Scholar]
- 38.Dai Z, Gorb SN, Schwarz U. 2002. Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J. Exp. Biol. 205, 2479–2488. [DOI] [PubMed] [Google Scholar]
- 39.Francius G, Tesson B, Dague E, Martin-Jezeuel V, Dufrene YF. 2008. Nanostructure and nanomechnics of live Phaeodactylum tricornutum morphotypes. Environ. Mircrobiol. 10, 1344–1356. ( 10.1111/j.1462-2920.2007.01551.x) [DOI] [PubMed] [Google Scholar]
- 40.Abe Y, Polyakov P, Skali-Lami S, Francius G. 2011. Elasticity and physico-chemical properties during drinking water biofilm formation. Biofouling 27, 739–750. ( 10.1080/08927014.2011.601300) [DOI] [PubMed] [Google Scholar]
- 41.Han SM, Saha R, Nix WD. 2006. Determining hardness of thin films in elastically mismatched film-on-substrate systems using nanoindentation. Acta Mater. 54, 1571–1581. ( 10.1016/j.actamat.2005.11.026) [DOI] [Google Scholar]
- 42.Hillerton JE, Reynolds SE, Vincent JF. 1982. On the indentation hardness of insect cuticle. J. Exp. Biol. 96, 44–52. [Google Scholar]
- 43.Lawrence JR, Scharf B, Packroff G, Neu TR. 2002. Microscale evaluation of the effects of grazing by invertebrates by contrasting feeding modus on river biofilm architecture and composition. Microb. Ecol. 43, 199–207. ( 10.1007/s00248-001-1064-y) [DOI] [PubMed] [Google Scholar]
- 44.Gonser T. 1990. Beiträge zur Biologie südneotropischer Ephemeropteren. PhD thesis, Albert-Ludwig Universität Freiburg, Freiburg, Germany. [Google Scholar]
- 45.Weissenberger J, Spatz HC, Emanns A, Schwoerbel J. 1991. Measurement of lift and drag forces in the mN range experienced by benthic arthropods at flow velocities below 1.2 m s−1. Freshw. Biol. 25, 21–31. ( 10.1111/j.1365-2427.1991.tb00469.x) [DOI] [Google Scholar]
- 46.Nachtigall W. 2000. Leben in der Grenzschicht—Festsitzende mikroskopische Organismen des Fließwassers nutzen eine physikalisch-ökologische Nische. Biol. Unserer Zeit 30, 148–157. () [DOI] [Google Scholar]
- 47.Vogel S. 1996. Life in moving fluids: the physical biology of flow. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.Statzner B. 2008. How views about flow adaptations of benthic stream invertebrate changed over the last century. Int. Rev. Hydrobiol. 93, 593–605. ( 10.1002/iroh.200711018) [DOI] [Google Scholar]