Abstract
Although it is now well known that some diseased areas, such as cancer nests, inflammation loci, and infarction areas, are acidified, little is known about cellular signal transduction, gene expression, and cellular functions under acidic conditions. Our group showed that different signal proteins were activated under acidic conditions compared with those observed in a typical medium of around pH 7.4 that has been used until now. Investigations of gene expression under acidic conditions may be crucial to our understanding of signal transduction in acidic diseased areas. In this study, we investigated gene expression in mesothelioma cells cultured at an acidic pH using a DNA microarray technique. After 24 h culture at pH 6.7, expressions of 379 genes were increased more than twofold compared with those in cells cultured at pH 7.5. Genes encoding receptors, signal proteins including transcription factors, and cytokines including growth factors numbered 35, 32, and 17 among the 379 genes, respectively. Since the functions of 78 genes are unknown, it can be argued that cells may have other genes for signaling under acidic conditions. The expressions of 37 of the 379 genes were observed to increase after as little as 2 h. After 24 h culture at pH 6.7, expressions of 412 genes were repressed more than twofold compared with those in cells cultured at pH 7.5, and the 412 genes contained 35, 76, and 7 genes encoding receptors, signal proteins including transcription factors, and cytokines including growth factors, respectively. These results suggest that the signal pathways in acidic diseased areas are different, at least in part, from those examined with cells cultured at a pH of around 7.4.
Keywords: gene expression, acidic conditions, signal pathways, cancer cells
1. Introduction
In mammals, the pH values of blood and tissues are usually maintained in a narrow range around 7.4 [1]. In contrast, diseased areas, such as cancer nests, inflammatory loci, and infarction areas, have been found to be acidic. The extracellular pH in the central regions of tumors decreases below 6.7 in several tumors as a consequence of lactate accumulation derived from a lack of sufficient vascularization or an increase in tumor-specific glycolysis under aerobic conditions combined with impaired mitochondrial oxidative phosphorylation [1,2,3]. Extracellular pH may also drop to a value below 6 due to leaking of intracellular contents and the destruction of blood vessels resulting in hypoxic metabolism and related lactic acid production during inflammation against the infection of pathogens [4]. Similar acidic environments were also associated with other inflammation. The pH value of articular fluid in the rheumatoid human knee joint was around 6.6, compared to around 7.3 in normal knee joints [5]. Other studies also showed the acidification of synovial fluid in arthritis [6,7,8].
Although cell functions mediated by a large number of enzymes with pH-dependent catalytic activity are strongly affected by the disruption of pH homeostasis, there have been only a few studies of signal transduction, gene expression, and cellular functions under acidic conditions. Studies of Escherichia coli have suggested that this bacterium has multiple systems for a single function and that different systems having optimum activities at different pH values function under different pH conditions [9,10].
Our group previously found that different signal transduction pathways function under acidic environments [11,12], and that CTIB, an IκB-β variant, acted as a critical factor at pH 6.3 but not at pH 7.4 [13,14]. Our group also showed the elevated activation of p38 and ERK in human T cells cultured at acidic pH [12,15]. In addition to these reports by our group, activation of the MAPK pathways and increased COX-2 protein expression were reported in acid exposed cells in Barrett’s metaplasia [16]. Matrix metalloproteinase-9 (MMP-9) expression was induced at acidic extracellular pH in mouse metastatic melanoma cells through phospholipase D-mitogen-activated protein kinase signaling [17]. Carbonic anhydrase 9 (CA9) expression was increased by acidosis via a hypoxia-independent mechanism that operates through modulation of the basic CA9 transcriptional machinery [18]. The gene expression of VEGF was stimulated at low extracellular pH [19,20]. Glioma stem cells grown in low pH conditions displayed an increase in expressions of Olig2, Oct4, Nanog, interleukin-8 (IL-8), TIMP1, TIMP2, VEGF, Glut1, SerpinB9, and HIF2α, whereas expressions of Sox2, GFAP, and HIF1α were repressed in the cells [21]. The expression of HIF1α induced by hypoxia was decreased by acidosis and the expression of ATF4 was increased by the combination of acidosis with hypoxia [22].
These previous findings led us to assume that different signal pathways operate under acidic conditions in mammalian cells. In addition to the molecules reported in previous studies described above, numerous molecules may work preferentially under low pH conditions. To exhaustively identify genes working for cell proliferation under acidic conditions, we used cancer cells that were able to proliferate rapidly and investigated the gene expression in mesothelioma cells cultured at acidic pH using a DNA microarray technique in the present study. After 24 h culture at pH 6.7, expressions of 379 genes were increased more than twofold compared with those in cells cultured at pH 7.5. The 379 genes contained 84 genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors, suggesting that the signal pathways in acidic diseased areas are different, at least in part, from those examined with cells cultured at pH around 7.4. The identified genes may be potential candidates for cancer chemotherapeutics. After 24 h culture at pH 6.7, expressions of 412 genes were repressed more than twofold compared with those in cells cultured at pH 7.5, and genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors numbered 118 among the 412 genes.
2. Materials and Methods
2.1. Cells and Medium for Their Maintenance
Human mesothelial cell line H2052, human colon adenocarcinoma grade II cell line HT-29, human esophageal cancer cell line TE-11, human pancreatic ductal adenocarcinoma cell line BxPC3, and human hepatocellular carcinoma cell line HepG2 were used. For cell maintenance, cells were cultured in RPMI-1640 (WAKO) containing 10 μg/mL gentamicin (Sigma), 1 μg/mL fungizone (Bristol-Myers), and 10% FBS (Sigma) in the presence of 5% CO2 at 37 °C.
2.2. Cell Culture under Different pH Conditions
Media for cell culture at various pH values were prepared as follows. To minimize the pH change during the cell culture, 10 mM PIPES [piperazine-N,N'-bis(ethanesulfonic acid)] for acidic media or HEPES [4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid] for alkaline media was added to RPMI-1640 instead of NaHCO3. Medium containing fetal bovine serum (FBS) was often contaminated with germs when the medium pH was adjusted, and it was hard to sterilize medium containing FBS. Therefore, medium pH was first adjusted by the addition of NaOH to 6.3 and 7.6 before the addition of FBS. After sterilization of the medium by filtration, FBS was added. The medium pH values were changed into 6.7 and 7.5 by the addition of FBS, respectively. Cells were cultured at 37 °C without a CO2 supply but with an air supply to avoid hypoxia and constant humidity.
2.3. DNA Microarray
After H2052 cells had been cultured in pH 7.5 medium as described above for 24 h at 37 °C, the medium was exchanged for pH 6.7 medium, and cells were cultured at 37 °C for 2, 5, and 24 h. Total RNA was isolated with the use of a TRI reagent (Sigma) according to the manufacturer’s instructions, and microarray analysis was entrusted to Roche Diagnostics Corporation using the Roche NimbleGen Microarray A4487001-00-01. In order to compare, data were processed using the NimbleScan software that was developed based on previous papers [23,24].
2.4. Real-Time Quantitative Polymerase Chain Reaction (PCR)
Total RNA (1 µg) prepared as described above was reverse-transcribed using ReverTra Ace (TOYOBO) in a total volume of 20 µL containing the random primer for 18S rRNA or the polyT primer for targeted genes. Real-time quantitative PCR amplification was performed with an ABI Prism 7000 Sequence Detection System (Applied Biosystems) using the FastStart Universal SYBR Green Master[Rox] (Roche Diagnostics) according to the manufacturer’s instructions. The PCR reaction was carried out with a mixture containing 12.5 µL of Real-Time PCR Master Mix, 7.5 µM of each sense and antisense primer, 25 ng of cDNA, and nuclease-free water in a total volume of 25 µL. The standard thermal profile for PCR amplification was 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 60 s. The primers used are shown in Table 1.
Table 1.
Primers used in this study.
| Gene name | Sequence | |
|---|---|---|
| 18S rRNA | F; | TAGAGTGTTCAAAGCAGGCCC |
| R; | CCAACAAATAGAACCGCGGT | |
| IL-32 | F; | TCAAAGAGGGCTACCTGGAG |
| R; | TTTCAAGTAGAGGAGTGAGCTCTG | |
| ATP6V0D2 | F; | GACCCAGCAAGACTATATCAACC |
| R; | TGGAGATGAATTTTCAGGTCTTC | |
| TNFRSF9 | F; | AAACGGGGCAGAAAGAAACT |
| R; | CTTCTGGAAATCGGCAGCTA | |
| AREG | F; | GGGAGTGAGATTTCCCCTGT |
| R; | AGCCAGGTATTTGTGGTTCG | |
| DMGDH | F; | GAGCTCACGGCTGGATCTAC |
| R; | CCACCACCTGACCAGTTTCT | |
| ERBB3 | F; | TGCAGTGGATTCGAGAAGTG |
| R; | GGCAAACTTCCCATCGTAGA | |
18S rRNA, 18S ribosomal ribonucleic acid; IL-32, interleukin 32; ATP6V0D2, V0 subunit d2 of lysosomal H+ transporting ATPase; TNFRSF9, tumor necrosis factor receptor superfamily member 9; AREG, amphiregulin; DMGDH, dimethylglycine dehydrogenase; ERBB3, erythroblastic leukemia viral oncogene homolog 3.
It has been reported that the content of ribosomes per cell is approximately 4 × 106 [25], and the amount of mRNA per cell can be estimated using 18S rRNA as a control RNA with the following equation.
| 4 × 106 × 2{(Ct of 18S rRNA) − (Ct of sample RNA)} |
where Ct is the threshold cycle number.
2.5. Other Reagents
Taq DNA polymerase (Bio Academia) and Ribonuclease inhibitor (TOYOBO) were used.
2.6. Statistical Analysis
The Student’s t-test was utilized in this study.
3. Results
3.1. Highly Expressed Genes under Acidic Conditions in Mesothelioma Cells
Approximately 24,000 genes were examined by microarray in mesothelioma cells (supplementary table), and the expressions of 379 genes were elevated more than twofold in cells cultured at pH 6.7 for 24 h compared with the cells cultured at pH 7.5 (Table 2). The accuracy of microarray analysis is mainly dependent on the RNA preparation. When the copy number of mRNA was low, the standard deviations of real-time quantitative PCR were close to 50% (Figure 1). We therefore assumed that more than twofold changes were significant in the present study. The 379 genes contained 35, 32, and 17 genes encoding receptors, signal proteins including transcription factors, and cytokines including growth factors, respectively (Table 2). The functions of 78 genes among the 379 genes are unknown.
Table 2.
Genes whose expression was induced more than twofold after 2, 5, and 24 h culture at acidic pH.
| Gene | 2 h | 5 h | 24 h |
|---|---|---|---|
| number of genes | 260 | 175 | 379 |
| receptors | 29 | 22 | 35 |
| signal proteins 1 | 25 | 21 | 32 |
| cytokines 2 | 5 | 10 | 17 |
1 including transcription factors; 2 including growth factors.
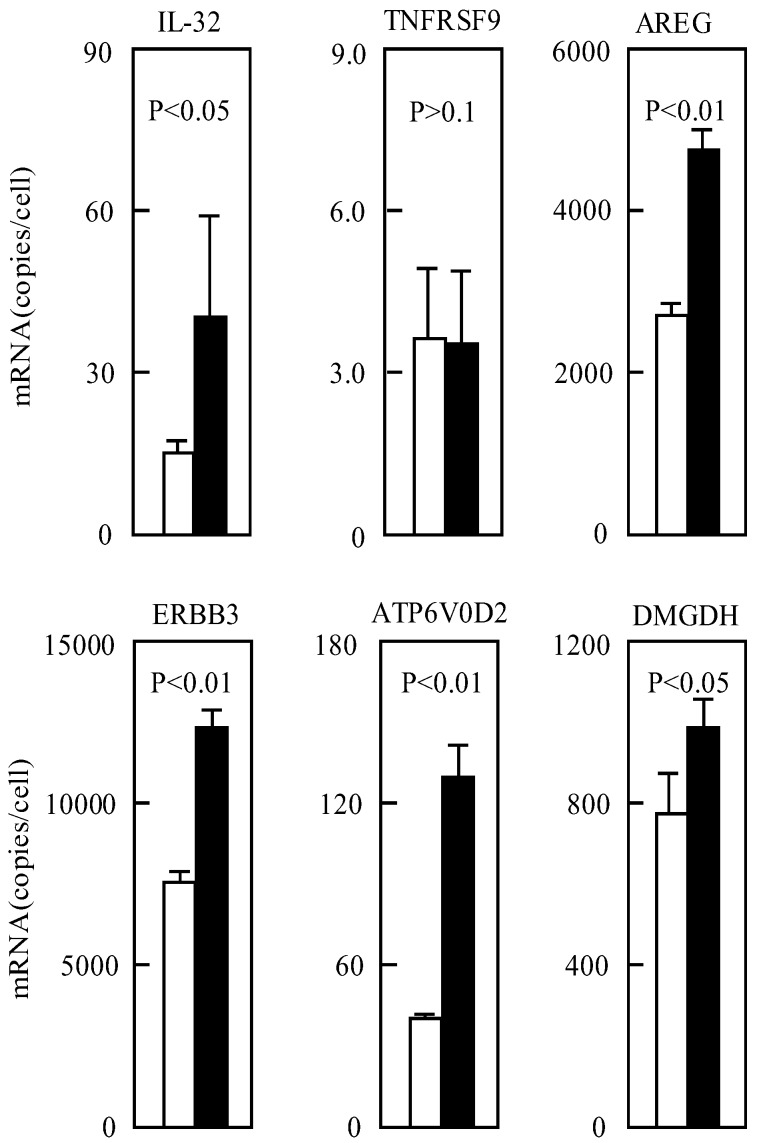
Figure 1.
Gene expressions of IL-32, TNFRSF9, AREG, ERBB3, ATP6V0D2, and DMGDH at pH 7.5 and 6.7 in TE-11 cells. TE-11 cells were incubated at pH 7.5 (open bars) and 6.7 (closed bars) for 24 h. mRNA number per one cell was detected with real-time quantitative PCR. Calculation is described in Materials and Methods. The mean values and standard deviations obtained from three independent experiments are represented. P values were calculated as described in Materials and Methods.
The expressions of IL-8 [21], MMP-9 [17], VEGF [19,20,21], CA9 [18], and COX-2 [16] were reported to increase under acidic stress. Our present results showed that the ratios of the expressions of IL-8, MMP-9, and VEGF in cells cultured at pH 6.7 to those at pH 7.5 were 2.52, 1.90, and 1.12, respectively. The expression of CA9 increased 1.35-fold at pH 6.7, but COX-2 expression was decreased at pH 6.7. It was reported that MnSOD participates in metastasis [26], and our data showed that the increase in the expression of MnSOD at acidic pH was 1.70-fold.
The previous reports by our group showed that p38 and ERK were activated more strongly at acidic pH than at alkaline pH [12,15]. The present data showed that p38-α (MAPK14) expression increased 1.71-fold after 5 h culture at pH 6.7, but decreased after 24 h culture at pH 6.7 (supplementary table), suggesting that p38-α is up-regulated for a short time after cells have been stressed by acidosis. The expressions of p38-γ (MAPK12) and ERK1 increased only 1.29-fold at pH 6.7. The expressions of other p38 and ERK families decreased slightly at acidic pH.
3.2. Gene Expression after Culture for a Short Period at pH 6.7
The gene expressions were also examined after 2 and 5 h at pH 6.7, and genes whose expression was increased were classified into seven groups as shown in Table 3. The expressions of 260 genes increased more than twofold in cells cultured at pH 6.7 for 2 h compared with pH 7.5. The 260 genes contained 29, 25, and 5 genes encoding receptors, signal proteins including transcription factors, and cytokines including growth factors, respectively (Table 2). The expressions of 15 among the 260 genes maintained high levels more than twofold for 24 h (Table 3, group A), while the expressions of 223 among the 260 genes decreased again after 24 h (Table 3, groups E and G). The 191 genes were expressed at a high level only at 2 h after the pH shift to 6.7 (Table 3, group G). After 5 h culture at pH 6.7, 175 genes were expressed more than twofold higher than the expression levels at pH 7.5 (Table 2), and the 91 genes were expressed at a high level only at 5 h after acidic stress (Table 3, group F). Genes encoding proteins for signal pathways among the genes whose expression was increased at acidic pH are listed in Table 4.
Table 3.
Classification of genes whose expression was induced at acidic pH.
| Group | Expression level * | Number of genes | |||
|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | Total | Signal ** | |
| A | >2 | >2 | >2 | 15 | 7 |
| B | >2 | <=2 | >2 | 22 | 3 |
| C | <=2 | >2 | >2 | 37 | 8 |
| D | <=2 | <=2 | >2 | 305 | 66 |
| E | >2 | >2 | <=2 | 32 | 6 |
| F | <=2 | >2 | <=2 | 91 | 32 |
| G | >2 | <=2 | <=2 | 191 | 43 |
| total | 693 | 165 | |||
* ratio of the expression in cells cultured at pH 6.7 to those at pH 7.5; ** genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors.
Table 4.
Genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors whose expression was high at pH 6.7.
| Gene | Ratio * | Accession number | Description |
|---|---|---|---|
| Group A | |||
| RSPO3 | 7.346 | NM_032784 | R-spondin 3 homolog (Xenopus laevis) |
| IL32 | 3.711 | NM_001012631 | interleukin 32 |
| TAS2R39 | 3.035 | NM_176881 | taste receptor, type 2, member 39 |
| SLAMF8 | 2.751 | NM_020125 | SLAM family member 8 |
| TRAF1 | 2.644 | NM_005658 | TNF receptor-associated factor 1 |
| IL8 | 2.519 | NM_000584 | interleukin 8 |
| RAB33A | 2.356 | NM_004794 | RAB33A, member RAS oncogene family |
| Group B | |||
| LOC553158 | 4.306 | NM_181334 | PRR5-ARHGAP8 fusion |
| PPP1R3E | 3.702 | XM_927029 | protein phosphatase 1, regulatory (inhibitor) subunit 3E |
| BDKRB2 | 2.168 | NM_000623 | bradykinin receptor B2 |
| Group C | |||
| TNFRSF9 | 5.464 | NM_001561 | tumor necrosis factor receptor superfamily, member 9 |
| FGF7 | 3.219 | NM_002009 | fibroblast growth factor 7 (keratinocyte growth factor) |
| ZNF226 | 2.926 | NM_015919 | zinc finger protein 226 |
| MGC17330 | 2.755 | NM_052880 | HGFL gene |
| IL1RAP | 2.551 | NM_134470 | interleukin 1 receptor accessory protein |
| NFKBIZ | 2.159 | NM_001005474 | nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, ζ |
| OLR1 | 2.054 | NM_002543 | oxidized low density lipoprotein (lectin-like) receptor 1 |
| TRIB3 | 2.031 | NM_021158 | tribbles homolog 3 (Drosophila) |
| Group D | |||
| ERBB3 | 5.997 | NM_001982 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (avian) |
| AREG | 5.650 | NM_001657 | amphiregulin (schwannoma-derived growth factor) |
| LOC653193 | 4.485 | XM_926448 | similar to Amphiregulin precursor (AR) (Colorectum cell-derived growth factor) (CRDGF) |
| RARRES1 | 3.882 | NM_002888 | retinoic acid receptor responder (tazarotene induced) 1 |
| RRAD | 3.827 | NM_004165 | Ras-related associated with diabetes |
| CRELD1 | 3.707 | NM_001031717 | cysteine-rich with EGF-like domains 1 |
| ARHGAP8 | 3.547 | NM_001017526 | Rho GTPase activating protein 8 |
| GPR78 | 3.302 | NM_080819 | G protein-coupled receptor 78 |
| GDF15 | 3.112 | NM_004864 | growth differentiation factor 15 |
| PTP4A3 | 3.037 | NM_007079 | protein tyrosine phosphatase type IVA, member 3 |
| IL16 | 2.926 | NM_004513 | interleukin 16 (lymphocyte chemoattractant factor) |
| PAQR6 | 2.919 | NM_198406 | progestin and adipoQ receptor family member VI |
| OR52N4 | 2.915 | NM_001005175 | olfactory receptor, family 52, subfamily N, member 4 |
| OR56B1 | 2.913 | NM_001005180 | olfactory receptor, family 56, subfamily B, member 1 |
| PTPRQ | 2.834 | XM_926134 | protein tyrosine phosphatase, receptor type, Q |
| LOC439957 | 2.784 | XM_495805 | similar to Ig κ chain V-I region Walker precursor |
| TNFSF9 | 2.744 | NM_003811 | tumor necrosis factor (ligand) superfamily, member 9 |
| TNFSF7 | 2.714 | NM_001252 | tumor necrosis factor (ligand) superfamily, member 7 |
| GPR87 | 2.641 | NM_023915 | G protein-coupled receptor 87 |
| Group D | |||
| GTF2IRD2B | 2.609 | NM_001003795 | general transcription factor 21 repeat domain containing 2β |
| RGS7 | 2.573 | NM_002924 | regulator of G-protein signalling 7 |
| FOLR3 | 2.506 | NM_000804 | folate receptor 3 (γ) |
| RELB | 2.471 | NM_006509 | v-rel reticuloendotheliosis viral oncogene homolog B, nuclear factor of κ light polypeptide gene enhancer in B-cells 3 (avian) |
| TAS2R40 | 2.459 | NM_176882 | taste receptor, type 2, member 40 |
| CCL3L3 | 2.418 | NM_001001437 | chemokine (C-C motif) ligand 3-like 3 |
| GPR144 | 2.391 | NM_182611 | G protein-coupled receptor 144 |
| RND1 | 2.389 | NM_014470 | Rho family GTPase 1 |
| CD6 | 2.381 | NM_006725 | CD6 molecule |
| ZNF165 | 2.368 | NM_003447 | zinc finger protein 165 |
| ICHTHYIN | 2.353 | XM_371777 | ichthyin protein |
| PKD1L1 | 2.334 | NM_138295 | polycystic kidney disease 1 like 1 |
| NPHP1 | 2.318 | NM_207181 | nephronophthisis 1 (juvenile) |
| PTK6 | 2.312 | NM_005975 | PTK6 protein tyrosine kinase 6 |
| IL15RA | 2.282 | NM_002189 | interleukin 15 receptor, α |
| POU6F1 | 2.271 | NM_002702 | POU domain, class 6, transcription factor 1 |
| TNFRSF10C | 2.268 | NM_003841 | tumor necrosis factor receptor superfamily, member 10c, decoy without an intracellular domain |
| IL15 | 2.248 | NM_172175 | interleukin 15 |
| P2RY12 | 2.233 | NM_176876 | purinergic receptor P2Y, G-protein coupled, 12 |
| MST1 | 2.186 | NM_020998 | macrophage stimulating 1 (hepatocyte growth factor-like) |
| KDR | 2.184 | NM_002253 | kinase insert domain receptor (a type III receptor tyrosine kinase) |
| GPR68 | 2.174 | NM_003485 | G protein-coupled receptor 68 |
| GPR44 | 2.170 | NM_004778 | G protein-coupled receptor 44 |
| RAI17 | 2.162 | NM_020338 | retinoic acid induced 17 |
| OR10V1 | 2.156 | NM_001005324 | olfactory receptor, family 10, subfamily V, member 1 |
| ASB1 | 2.148 | NM_016114 | ankyrin repeat and SOCS box-containing 1 |
| CMTM1 | 2.146 | NM_181293 | CKLF-like MARVEL transmembrane domain containing 1 |
| PHF7 | 2.141 | NM_173341 | PHD finger protein 7 |
| GPRC5D | 2.114 | NM_018654 | G protein-coupled receptor, family C, group 5, member D |
| TP53INP2 | 2.108 | NM_021202 | tumor protein p53 inducible nuclear protein 2 |
| ARHGAP15 | 2.082 | NM_018460 | Rho GTPase activating protein 15 |
| GEFT | 2.066 | NM_182947 | RAC/CDC42 exchange factor |
| PIM1 | 2.062 | NM_002648 | pim-1 oncogene |
| TNFRSF25 | 2.055 | NM_148973 | tumor necrosis factor receptor superfamily, member 25 |
| GPR157 | 2.049 | NM_024980 | G protein-coupled receptor 157 |
| NR2E3 | 2.045 | NM_014249 | nuclear receptor subfamily 2, group E, member 3 |
| LOC619207 | 2.042 | XM_927510 | scavenger receptor protein family member |
| WISP3 | 2.033 | NM_003880 | WNT1 inducible signaling pathway protein 3 |
| P2RX4 | 2.030 | NM_002560 | purinergic receptor P2X, ligand-gated ion channel, 4 |
| RASD2 | 2.029 | NM_014310 | RASD family, member 2 |
| FGF2 | 2.028 | NM_002006 | fibroblast growth factor 2 (basic) |
| RGR | 2.011 | NM_001012720 | retinal G protein coupled receptor |
| Group D | |||
| NRXN2 | 2.011 | NM_015080 | neurexin 2 |
| EDG4 | 2.009 | NM_004720 | endothelial differentiation, lysophosphatidic acid G-protein-coupled receptor, 4 |
| KGFLP1 | 2.006 | NM_174950 | keratinocyte growth factor-like protein 1 |
| PTPRH | 2.005 | NM_002842 | protein tyrosine phosphatase, receptor type, H |
| OR52A5 | 2.001 | NM_001005160 | olfactory receptor, family 52, subfamily A, member 5 |
| Group E | |||
| KLF9 | 3.169 | NM_001206 | Kruppel-like factor 9 |
| CLASP2 | 2.029 | NM_015097 | cytoplasmic linker associated protein 2 |
| E2F5 | 2.267 | NM_001951 | E2F transcription factor 5, p130-binding |
| ZNF474 | 2.203 | NM_207317 | zinc finger protein 474 |
| GPR37 | 2.411 | NM_005302 | G protein-coupled receptor 37 (endothelin receptor type B-like) |
| PAX5 | 2.097 | NM_016734 | paired box gene 5 (B-cell lineage specific activator) |
| Group F | |||
| OR4D6 | 2.809 | NM_001004708 | olfactory receptor, family 4, subfamily D, member 6 |
| OR5B12 | 2.783 | NM_001004733 | olfactory receptor, family 5, subfamily B, member 12 |
| VENTX | 2.515 | NM_014468 | VENT homeobox homolog (Xenopus laevis) |
| UNC5B | 2.438 | NM_170744 | unc-5 homolog B (C. elegans) |
| OR1J4 | 2.416 | NM_001004452 | olfactory receptor, family 1, subfamily J, member 4 |
| NR5A1 | 2.338 | NM_004959 | nuclear receptor subfamily 5, group A, member 1 |
| SESN2 | 2.331 | NM_031459 | sestrin 2 |
| CCL25 | 2.308 | NM_148888 | chemokine (C-C motif) ligand 25 |
| IL21R | 2.301 | NM_021798 | interleukin 21 receptor |
| ATF3 | 2.220 | NM_001030287 | activating transcription factor 3 |
| TLR1 | 2.212 | NM_003263 | toll-like receptor 1 |
| C1QTNF7 | 2.202 | NM_031911 | C1q and tumor necrosis factor related protein 7 |
| TBX19 | 2.186 | NM_005149 | T-box 19 |
| MXD1 | 2.163 | NM_002357 | MAX dimerization protein 1 |
| GTPBP2 | 2.148 | NM_019096 | GTP binding protein 2 |
| NR4A2 | 2.117 | NM_006186 | nuclear receptor subfamily 4, group A, member 2 |
| PHLDA1 | 2.116 | NM_007350 | pleckstrin homology-like domain, family A, member 1 |
| LCP1 | 2.111 | NM_002298 | lymphocyte cytosolic protein 1 (L-plastin) |
| FGFBP1 | 2.111 | NM_005130 | fibroblast growth factor binding protein 1 |
| OR2B11 | 2.078 | NM_001004492 | olfactory receptor, family 2, subfamily B, member 11 |
| OR56A3 | 2.073 | NM_001003443 | olfactory receptor, family 56, subfamily A, member 3 |
| GH2 | 2.071 | NM_002059 | growth hormone 2 |
| PTHLH | 2.061 | NM_002820 | parathyroid hormone-like hormone |
| THBD | 2.059 | NM_000361 | thrombomodulin |
| HGF | 2.055 | NM_000601 | hepatocyte growth factor (hepapoietin A; scatter factor) |
| ARTN | 2.051 | NM_003976 | artemin |
| EPHA8 | 2.040 | NM_020526 | EPH receptor A8 |
| CD200R1 | 2.023 | NM_138939 | CD200 receptor 1 |
| FZD7 | 2.017 | NM_003507 | frizzled homolog 7 (Drosophila) |
| S100A12 | 2.012 | NM_005621 | S100 calcium binding protein A12 (calgranulin C) |
| Group F | |||
| SPIC | 2.008 | NM_152323 | Spi-C transcription factor (Spi-1/PU.1 related) |
| VSIG4 | 2.006 | NM_007268 | V-set and immunoglobulin domain containing 4 |
| Group G | |||
| TCF21 | 3.518 | NM_198392 | transcription factor 21 |
| DOK6 | 2.827 | NM_152721 | docking protein 6 |
| FZD3 | 2.815 | NM_017412 | frizzled homolog 3 (Drosophila) |
| CD86 | 2.686 | NM_006889 | CD86 molecule |
| OR2L13 | 2.677 | NM_175911 | olfactory receptor, family 2, subfamily L, member 13 |
| IL18RAP | 2.557 | NM_003853 | interleukin 18 receptor accessory protein |
| TRPA1 | 2.427 | NM_007332 | transient receptor potential cation channel, subfamily A, member 1 |
| RTP3 | 2.417 | NM_031440 | receptor transporter protein 3 |
| GRIA2 | 2.353 | NM_000826 | glutamate receptor, ionotropic, AMPA 2 |
| OR51E1 | 2.351 | NM_152430 | olfactory receptor, family 51, subfamily E, member 1 |
| GRM2 | 2.343 | NM_000839 | glutamate receptor, metabotropic 2 |
| FCRLM1 | 2.335 | NM_032738 | Fc receptor-like and mucin-like 1 |
| NR4A3 | 2.332 | NM_173199 | nuclear receptor subfamily 4, group A, member 3 |
| SPI1 | 2.288 | NM_003120 | spleen focus forming virus (SFFV) proviral integration oncogene spi1 |
| SMAD6 | 2.282 | NM_005585 | SMAD, mothers against DPP homolog 6 (Drosophila) |
| LOC642400 | 2.281 | XM_925921 | similar to tripartite motif protein 17 |
| CAMTA1 | 2.274 | NM_015215 | calmodulin binding transcription activator 1 |
| GDF6 | 2.260 | NM_001001557 | growth differentiation factor 6 |
| OR51B2 | 2.258 | NM_033180 | olfactory receptor, family 51, subfamily B, member 2 |
| OR7D4 | 2.238 | NM_001005191 | olfactory receptor, family 7, subfamily D, member 4 |
| ECGF1 | 2.227 | NM_001953 | endothelial cell growth factor 1 (platelet-derived) |
| LAIR1 | 2.218 | NM_002287 | leukocyte-associated immunoglobulin-like receptor 1 |
| NELL1 | 2.209 | NM_006157 | NEL-like 1 (chicken) |
| OR8J1 | 2.188 | NM_001005205 | olfactory receptor, family 8, subfamily J, member 1 |
| GRM3 | 2.178 | NM_000840 | glutamate receptor, metabotropic 3 |
| PRKCQ | 2.170 | NM_006257 | protein kinase C, θ |
| PPP1R3F | 2.164 | NM_033215 | protein phosphatase 1, regulatory (inhibitor) subunit 3F |
| LOC642338 | 2.150 | XM_925874 | similar to vomeronasal 1 receptor, C3 |
| CPNE5 | 2.148 | NM_020939 | copine V |
| EPHB6 | 2.134 | NM_004445 | EPH receptor B6 |
| OR51M1 | 2.119 | NM_001004756 | olfactory receptor, family 51, subfamily M, member 1 |
| PTPRC | 2.105 | NM_002838 | protein tyrosine phosphatase, receptor type, C |
| EBF3 | 2.100 | NM_001005463 | early B-cell factor 3 |
| BMPR1B | 2.091 | NM_001203 | bone morphogenetic protein receptor, type IB |
| HRH4 | 2.084 | NM_021624 | histamine receptor H4 |
| SHE | 2.082 | NM_001010846 | Src homology 2 domain containing E |
| T2R55 | 2.073 | NM_181429 | taste receptor T2R55 |
| SBK1 | 2.067 | NM_001024401 | SH3-binding domain kinase 1 |
| RASGRP2 | 2.048 | NM_005825 | RAS guanyl releasing protein 2 (calcium and DAG-regulated) |
| CD96 | 2.047 | NM_005816 | CD96 molecule |
| GRIN2B | 2.028 | NM_000834 | glutamate receptor, ionotropic, N-methyl D-aspartate 2B |
| Group G | |||
| YAF2 | 2.018 | NM_001012424 | YY1 associated factor 2 |
| LCP2 | 2.001 | NM_005565 | lymphocyte cytosolic protein 2 (SH2 domain containing leukocyte protein of 76 kDa) |
* ratio of the expression in cells cultured at pH 6.7 after 24, 5, and 2 h culture to those at pH 7.5 in groups A to D, E to F, and G, respectively.
3.3. Genes Whose Expression Was Repressed at Acidic pH
The expressions of 412 genes were repressed more than twofold in cells cultured at pH 6.7 for 24 h, and the 412 genes contained 35, 76, and 7 genes encoding receptors, signal proteins including transcription factors, and cytokines including growth factors, respectively (Table 5). Genes whose expression was repressed at acidic pH were classified into seven groups, as shown in Table 6. The expressions of 17 genes decreased already after 2 h culture at pH 6.7 (Table 6, groups A and B).
Table 5.
Genes whose expression was repressed more than twofold after 2, 5, and 24 h culture at acidic pH.
| Gene | 2 h | 5 h | 24 h |
|---|---|---|---|
| number of genes | 385 | 141 | 412 |
| receptors | 32 | 14 | 35 |
| signal proteins 1 | 31 | 14 | 76 |
| cytokines 2 | 8 | 0 | 7 |
1 including transcription factors; 2 including growth factors.
Table 6.
Classification of genes whose expression was repressed at acidic pH.
| Group | Expression level * | Number of genes | |||
|---|---|---|---|---|---|
| 2 h | 5 h | 24 h | Total | Signal ** | |
| A | <0.5 | <0.5 | <0.5 | 8 | 4 |
| B | <0.5 | >=0.5 | <0.5 | 9 | 3 |
| C | >=0.5 | <0.5 | <0.5 | 32 | 4 |
| D | >=0.5 | >=0.5 | <0.5 | 363 | 107 |
| E | <0.5 | <0.5 | >=0.5 | 25 | 8 |
| F | >=0.5 | <0.5 | >=0.5 | 76 | 12 |
| G | <0.5 | >=0.5 | >=0.5 | 343 | 56 |
| total | 856 | 194 | |||
* ratio of the expression in cells cultured at pH 6.7 to those at pH 7.5; ** genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors.
The expressions of 385 genes were repressed more than twofold in cells cultured at pH 6.7 for 2 h (Table 5), but the expressions of 368 of these 385 genes increased again after 24 h culture (Table 6, groups E and G). The expressions of 343 among 385 genes were repressed only after 2 h culture at pH 6.7 (Table 6, group G). After 5 h culture at pH 6.7, the expressions of 141 genes were repressed (Table 5) and 76 genes were repressed only after 5 h culture at pH 6.7 (Table 6, group F). Genes encoding proteins for signal pathways among the genes whose expression was repressed at acidic pH are listed in Table 7.
Table 7.
Genes encoding receptors, signal proteins, transcription factors, cytokines, and growth factors whose expression was repressed at pH 6.7.
| Gene | Ratio * | Accession number | Description |
|---|---|---|---|
| Group A | |||
| IL11 | 0.158 | NM_000641 | interleukin 11 |
| CCRL2 | 0.324 | NM_003965 | chemokine (C-C motif) receptor-like 2 |
| CD300LG | 0.444 | NM_145273 | CD300 molecule-like family member g |
| ATOH1 | 0.459 | NM_005172 | atonal homolog 1 (Drosophila) |
| Group B | |||
| RASGEF1C | 0.486 | NM_001031799 | RasGEF domain family, member 1C |
| LGR5 | 0.491 | NM_003667 | leucine-rich repeat-containing G protein-coupled receptor 5 |
| HSH2D | 0.493 | NM_032855 | hematopoietic SH2 domain containing |
| Group C | |||
| TLR4 | 0.099 | NM_138554 | toll-like receptor 4 |
| TSSK2 | 0.421 | NM_053006 | testis-specific serine kinase 2 |
| ADRB2 | 0.475 | NM_000024 | adrenergic, β-2-, receptor, surface |
| FLRT2 | 0.400 | NM_013231 | fibronectin leucine rich transmembrane protein 2 |
| Group D | |||
| E2F2 | 0.150 | NM_004091 | E2F transcription factor 2 |
| ADRA2A | 0.193 | NM_000681 | adrenergic, α-2A-, receptor |
| APLN | 0.243 | NM_017413 | apelin, AGTRL1 ligand |
| REEP1 | 0.244 | NM_022912 | receptor accessory protein 1 |
| ARHGAP26 | 0.252 | NM_015071 | Rho GTPase activating protein 26 |
| UHRF1 | 0.259 | NM_013282 | ubiquitin-like, containing PHD and RING finger domains, 1 |
| ZNF367 | 0.261 | NM_153695 | zinc finger protein 367 |
| POU5F1 | 0.267 | NM_203289 | POU domain, class 5, transcription factor 1 |
| RGS4 | 0.269 | NM_005613 | regulator of G-protein signalling 4 |
| RHOJ | 0.269 | NM_020663 | ras homolog gene family, member J |
| MCF2 | 0.270 | NM_005369 | MCF.2 cell line derived transforming sequence |
| CHRNA5 | 0.276 | NM_000745 | cholinergic receptor, nicotinic, α 5 |
| GPR115 | 0.285 | NM_153838 | G protein-coupled receptor 115 |
| SORCS3 | 0.286 | NM_014978 | sortilin-related VPS10 domain containing receptor 3 |
| RBM14 | 0.287 | NM_006328 | RNA binding motif protein 14 |
| PDE4B | 0.298 | NM_001037339 | phosphodiesterase 4B, cAMP-specific (phosphodiesterase E4 dunce homolog, Drosophila) |
| PIK3CG | 0.310 | NM_002649 | phosphoinositide-3-kinase, catalytic, γ polypeptide |
| RGPD2 | 0.311 | NM_001024457 | RANBP2-like and GRIP domain containing 2 |
| TP53RK | 0.314 | NM_033550 | TP53 regulating kinase |
| MAP2K6 | 0.320 | NM_002758 | mitogen-activated protein kinase kinase 6 |
| TP73 | 0.330 | NM_005427 | tumor protein p73 |
| GPR63 | 0.338 | NM_030784 | G protein-coupled receptor 63 |
| FST | 0.340 | NM_006350 | follistatin |
| MPP4 | 0.347 | NM_033066 | membrane protein, palmitoylated 4 (MAGUK p55 subfamily member 4) |
| PDE4D | 0.350 | NM_006203 | phosphodiesterase 4D, cAMP-specific (phosphodiesterase E3 dunce homolog, Drosophila) |
| ANXA10 | 0.355 | NM_007193 | annexin A10 |
| Group D | |||
| RBL1 | 0.355 | NM_002895 | retinoblastoma-like 1 (p107) |
| KIT | 0.360 | NM_000222 | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog |
| PBX1 | 0.368 | NM_002585 | pre-B-cell leukemia transcription factor 1 |
| MTUS1 | 0.371 | NM_001001924 | mitochondrial tumor suppressor 1 |
| RORB | 0.386 | NM_006914 | RAR-related orphan receptor B |
| LHX6 | 0.389 | NM_014368 | LIM homeobox 6 |
| PAQR4 | 0.392 | NM_152341 | progestin and adipoQ receptor family member IV |
| ABRA | 0.394 | NM_139166 | actin-binding Rho activating protein |
| GDAP1 | 0.396 | NM_018972 | ganglioside-induced differentiation-associated protein 1 |
| C1QTNF2 | 0.399 | NM_031908 | C1q and tumor necrosis factor related protein 2 |
| CMTM1 | 0.400 | NM_181289 | CKLF-like MARVEL transmembrane domain containing 1 |
| MLR1 | 0.404 | NM_153686 | transcription factor MLR1 |
| TSPAN8 | 0.405 | NM_004616 | tetraspanin 8 |
| SH2D4B | 0.406 | NM_207372 | SH2 domain containing 4B |
| E2F1 | 0.406 | NM_005225 | E2F transcription factor 1 |
| VANGL1 | 0.411 | NM_138959 | vang-like 1 (van gogh, Drosophila) |
| DUSP6 | 0.415 | NM_001946 | dual specificity phosphatase 6 |
| FZD3 | 0.416 | NM_017412 | frizzled homolog 3 (Drosophila) |
| PPARGC1A | 0.417 | NM_013261 | peroxisome proliferative activated receptor, γ, coactivator 1, α |
| HOXB7 | 0.419 | NM_004502 | homeobox B7 |
| PTGER2 | 0.420 | NM_000956 | prostaglandin E receptor 2 (subtype EP2), 53 kDa |
| NGEF | 0.421 | NM_019850 | neuronal guanine nucleotide exchange factor |
| FGF18 | 0.421 | NM_033649 | fibroblast growth factor 18 |
| LOC653528 | 0.425 | XM_927910 | similar to Teratocarcinoma-derived growth factor 2 (Epidermal growth factor-like cripto protein CR3) (Cripto-3 growth factor) |
| OR4N2 | 0.426 | NM_001004723 | olfactory receptor, family 4, subfamily N, member 2 |
| NKX6-2 | 0.429 | NM_177400 | NK6 transcription factor related, locus 2 (Drosophila) |
| NFKBIL2 | 0.431 | NM_013432 | nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor-like 2 |
| PTPN22 | 0.431 | NM_012411 | protein tyrosine phosphatase, non-receptor type 22 (lymphoid) |
| LOC392269 | 0.432 | XM_928112 | similar to Transcription factor SOX-2 |
| MAL2 | 0.432 | NM_052886 | mal, T-cell differentiation protein 2 |
| SELPLG | 0.434 | NM_003006 | selectin P ligand |
| GPR177 | 0.434 | NM_001002292 | G protein-coupled receptor 177 |
| NCOA5 | 0.437 | NM_020967 | nuclear receptor coactivator 5 |
| RIF1 | 0.437 | NM_018151 | RAP1 interacting factor homolog (yeast) |
| GPR3 | 0.439 | NM_005281 | G protein-coupled receptor 3 |
| CDC14A | 0.439 | NM_003672 | CDC14 cell division cycle 14 homolog A (S. cerevisiae) |
| RP3-509I19.5 | 0.444 | XM_294019 | similar to ECT2 protein (Epithelial cell transforming sequence 2 oncogene) |
| ADORA1 | 0.444 | NM_000674 | adenosine A1 receptor |
| PTCH | 0.446 | NM_000264 | patched homolog (Drosophila) |
| TCF21 | 0.446 | NM_003206 | transcription factor 21 |
| SPRY4 | 0.448 | NM_030964 | sprouty homolog 4 (Drosophila) |
| CBX2 | 0.450 | NM_005189 | chromobox homolog 2 (Pc class homolog, Drosophila) |
| OR6C74 | 0.451 | NM_001005490 | olfactory receptor, family 6, subfamily C, member 74 |
| Group D | |||
| CXCL14 | 0.452 | NM_004887 | chemokine (C-X-C motif) ligand 14 |
| CUBN | 0.453 | NM_001081 | cubilin (intrinsic factor-cobalamin receptor) |
| NRG2 | 0.457 | NM_013985 | neuregulin 2 |
| SGIP1 | 0.457 | NM_032291 | SH3-domain GRB2-like (endophilin) interacting protein 1 |
| GNGT2 | 0.457 | NM_031498 | guanine nucleotide binding protein (G protein), γ transducing activity polypeptide 2 |
| EBF | 0.458 | NM_024007 | early B-cell factor |
| ACVR1C | 0.458 | NM_145259 | activin A receptor, type IC |
| PHTF2 | 0.458 | NM_020432 | putative homeodomain transcription factor 2 |
| RASSF1 | 0.460 | NM_007182 | Ras association (RalGDS/AF-6) domain family 1 |
| GPR109A | 0.462 | NM_177551 | G protein-coupled receptor 109A |
| TSHR | 0.463 | NM_000369 | thyroid stimulating hormone receptor |
| SIM2 | 0.468 | NM_009586 | single-minded homolog 2 (Drosophila) |
| GABRA6 | 0.469 | NM_000811 | γ-aminobutyric acid (GABA) A receptor, alpha 6 |
| LAT2 | 0.469 | NM_032464 | linker for activation of T cells family, member 2 |
| PHKG1 | 0.472 | NM_006213 | phosphorylase kinase, γ 1 (muscle) |
| RGPD4 | 0.473 | XM_496581 | RANBP2-like and GRIP domain containing 4 |
| NKD1 | 0.474 | NM_033119 | naked cuticle homolog 1 (Drosophila) |
| ZNF588 | 0.475 | NM_001013746 | zinc finger protein 588 |
| SH3TC2 | 0.476 | NM_024577 | SH3 domain and tetratricopeptide repeats 2 |
| FZD1 | 0.478 | NM_003505 | frizzled homolog 1 (Drosophila) |
| PKMYT1 | 0.478 | NM_004203 | protein kinase, membrane associated tyrosine/threonine 1 |
| DUSP4 | 0.480 | NM_001394 | dual specificity phosphatase 4 |
| WDR4 | 0.480 | NM_018669 | WD repeat domain 4 |
| WDR76 | 0.481 | NM_024908 | WD repeat domain 76 |
| WDHD1 | 0.483 | NM_001008396 | WD repeat and HMG-box DNA binding protein 1 |
| HOXA7 | 0.485 | NM_006896 | homeobox A7 |
| WDR69 | 0.486 | NM_178821 | WD repeat domain 69 |
| TFAP2C | 0.487 | NM_003222 | transcription factor AP-2 γ (activating enhancer binding protein 2 γ) |
| CDGAP | 0.488 | NM_020754 | Cdc42 GTPase-activating protein |
| RPIB9 | 0.491 | NM_138290 | Rap2-binding protein 9 |
| IFNAR1 | 0.493 | NM_000629 | interferon (α, β and ω) receptor 1 |
| POU3F2 | 0.493 | NM_005604 | POU domain, class 3, transcription factor 2 |
| LOC402279 | 0.497 | XM_377945 | similar to glutamate receptor, metabotropic 8 |
| EYA4 | 0.498 | NM_004100 | eyes absent homolog 4 (Drosophila) |
| ISL1 | 0.498 | NM_002202 | ISL1 transcription factor, LIM/homeodomain, (islet-1) |
| SIRPD | 0.499 | NM_178460 | signal-regulatory protein δ |
| NEDD9 | 0.499 | NM_182966 | neural precursor cell expressed, developmentally down-regulated 9 |
| TLR3 | 0.500 # | NM_003265 | toll-like receptor 3 |
| Group E | |||
| RAPSN | 0.354 | NM_005055 | receptor-associated protein of the synapse, 43 kDa |
| GRAP | 0.363 | NM_006613 | GRB2-related adaptor protein |
| CD48 | 0.410 | NM_001778 | CD48 molecule |
| LOC642966 | 0.428 | XM_926351 | similar to olfactory receptor 139 |
| SALL1 | 0.437 | NM_002968 | sal-like 1 (Drosophila) |
| Group E | |||
| GLIS1 | 0.438 | NM_147193 | GLIS family zinc finger 1 |
| FOLR1 | 0.471 | NM_016725 | folate receptor 1 (adult) |
| NRG4 | 0.482 | NM_138573 | neuregulin 4 |
| Group F | |||
| TACR1 | 0.408 | NM_015727 | tachykinin receptor 1 |
| NHLH1 | 0.414 | NM_005598 | nescient helix loop helix 1 |
| NF2 | 0.420 | NM_181825 | neurofibromin 2 (bilateral acoustic neuroma) |
| LOC440607 | 0.427 | NM_001004340 | Fc-γ receptor I B2 |
| MAF | 0.443 | NM_001031804 | v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) |
| ZNF160 | 0.449 | NM_033288 | zinc finger protein 160 |
| DUSP2 | 0.454 | NM_004418 | dual specificity phosphatase 2 |
| SOCS1 | 0.456 | NM_003745 | suppressor of cytokine signaling 1 |
| CHRNA3 | 0.471 | NM_000743 | cholinergic receptor, nicotinic, α 3 |
| CRLF2 | 0.481 | NM_022148 | cytokine receptor-like factor 2 |
| MRAP | 0.487 | NM_178817 | melanocortin 2 receptor accessory protein |
| RPIP8 | 0.491 | NM_006695 | RaP2 interacting protein 8 |
| Group G | |||
| CLEC4G | 0.198 | NM_198492 | C-type lectin superfamily 4, member G |
| FSTL4 | 0.246 | NM_015082 | follistatin-like 4 |
| RAB6C | 0.282 | NM_032144 | RAB6C, member RAS oncogene family |
| CSF3 | 0.295 | NM_172220 | colony stimulating factor 3 (granulocyte) |
| OR2T34 | 0.301 | NM_001001821 | olfactory receptor, family 2, subfamily T, member 34 |
| RRP22 | 0.304 | NM_001007279 | RAS-related on chromosome 22 |
| UTF1 | 0.305 | NM_003577 | undifferentiated embryonic cell transcription factor 1 |
| CHRND | 0.306 | NM_000751 | cholinergic receptor, nicotinic, δ |
| GPR6 | 0.316 | NM_005284 | G protein-coupled receptor 6 |
| ANGPTL6 | 0.324 | NM_031917 | angiopoietin-like 6 |
| OR2M7 | 0.346 | NM_001004691 | olfactory receptor, family 2, subfamily M, member 7 |
| OR10P1 | 0.356 | NM_206899 | olfactory receptor, family 10, subfamily P, member 1 |
| FOXD3 | 0.364 | NM_012183 | forkhead box D3 |
| ZAP70 | 0.377 | NM_207519 | ζ-chain (TCR) associated protein kinase 70 kDa |
| PTGER3 | 0.392 | NM_000957 | prostaglandin E receptor 3 (subtype EP3) |
| CDX4 | 0.395 | NM_005193 | caudal type homeobox transcription factor 4 |
| TBX21 | 0.405 | NM_013351 | T-box 21 |
| TAS2R13 | 0.408 | NM_023920 | taste receptor, type 2, member 13 |
| IL17RE | 0.414 | NM_153482 | interleukin 17 receptor E |
| PRDM9 | 0.415 | NM_020227 | PR domain containing 9 |
| CXCL12 | 0.422 | NM_199168 | chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) |
| LILRA4 | 0.430 | NM_012276 | leukocyte immunoglobulin-like receptor, subfamily A (with TM domain), member 4 |
| LOC642506 | 0.433 | XM_926003 | similar to double homeobox 4c |
| NEUROD6 | 0.435 | NM_022728 | neurogenic differentiation 6 |
| KLF14 | 0.438 | NM_138693 | Kruppel-like factor 14 |
| TFAP2E | 0.439 | NM_178548 | transcription factor AP-2 ε (activating enhancer binding protein 2 ε) |
| Group G | |||
| CCL1 | 0.439 | NM_002981 | chemokine (C-C motif) ligand 1 |
| VAV3 | 0.439 | NM_006113 | vav 3 oncogene |
| IRS3L | 0.444 | XM_498229 | insulin receptor substrate 3-like |
| GPR81 | 0.445 | NM_032554 | G protein-coupled receptor 81 |
| GPR32 | 0.445 | NM_001506 | G protein-coupled receptor 32 |
| GDF7 | 0.446 | NM_182828 | growth differentiation factor 7 |
| WDR42C | 0.447 | XM_293354 | WD repeat domain 42C |
| LOC619207 | 0.454 | XM_927516 | scavenger receptor protein family member |
| FOLR1 | 0.457 | NM_016724 | folate receptor 1 (adult) |
| ADRA1D | 0.457 | NM_000678 | adrenergic, α-1D-, receptor |
| IL12RB2 | 0.459 | NM_001559 | interleukin 12 receptor, β 2 |
| GRIN1 | 0.460 | NM_007327 | glutamate receptor, ionotropic, N-methyl D-aspartate 1 |
| SHC2 | 0.461 | XM_375550 | SHC (Src homology 2 domain containing) transforming protein 2 |
| RAXL1 | 0.464 | NM_032753 | retina and anterior neural fold homeobox like 1 |
| CAMK2B | 0.472 | NM_172084 | calcium/calmodulin-dependent protein kinase (CaM kinase) II β |
| CCL15 | 0.473 | NM_004167 | chemokine (C-C motif) ligand 15 |
| FSHR | 0.474 | NM_000145 | follicle stimulating hormone receptor |
| WDR40B | 0.478 | NM_178470 | WD repeat domain 40B |
| MAFB | 0.482 | NM_005461 | v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (avian) |
| TPRX1 | 0.484 | NM_198479 | tetra-peptide repeat homeobox 1 |
| FLT1 | 0.487 | NM_002019 | fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) |
| OLIG2 | 0.488 | NM_005806 | oligodendrocyte lineage transcription factor 2 |
| TBXA2R | 0.490 | NM_001060 | thromboxane A2 receptor |
| SSTR5 | 0.490 | NM_001053 | somatostatin receptor 5 |
| MYOG | 0.491 | NM_002479 | myogenin (myogenic factor 4) |
| OR2AG1 | 0.492 | NM_001004489 | olfactory receptor, family 2, subfamily AG, member 1 |
| FOXD4L1 | 0.495 | NM_012184 | forkhead box D4-like 1 |
| PSPN | 0.495 | NM_004158 | persephin |
| PJCG6 | 0.496 | NM_001040066 | similar to olfactory receptor 873 |
| TSHR | 0.499 | NM_001018036 | thyroid stimulating hormone receptor |
* ratio of the expression in cells cultured at pH 6.7 after 24, 5, and 2 h culture to those at pH 7.5 in groups A to D, E to F, and G, respectively; # 0.499857.
3.4. The Gene Expressions in Various Cells
To confirm whether or not the gene expression pattern observed in mesothelioma cells is specific to these cells, we selected six genes, IL-32, TNFRSF9, AREG, ERBB3, ATP6V0D2, and DMGDH whose expressions were observed to increase more than three-fold at acidic pH using a microarray, and examined their expressions in various cells. IL-32 has been reported to be a cytokine, but its function remains unclear. TNFRSF9, AREG, and ERBB3 have been reported to be a receptor, growth factor, and oncogene product, respectively. ATP6V0D2 is one subunit of ATPase which has a role in pH regulation. DMGDH is a mitochondrial matrix enzyme.
One problem in the measurement of mRNA was determining which gene was available as a control gene. A housekeeping gene such as GAPDH has been used generally until now. The previous report from our group showed that the amount of 18S rRNA was constant at both acidic and alkaline pH in human T cells [15]. The amount of 18S rRNA in mesothelioma cells did not vary as pH changed (data not shown). Based on these data, 18S rRNA was used as a control RNA in this study. The amount of ribosomes per cell was approximately 4 × 106 [25]. The copy number of mRNA per cell can be estimated using this number.
IL-32, AREG, ERBB3, ATP6V0D2, and DMGDH genes showed increased expression in TE-11 cells after 24 h culture at acidic pH (Figure 1). These data were in agreement with the DNA array data. In contrast, the expression of TNFRSF9 did not increase under acidic pH in TE-11 (Figure 1).
The expression of IL-32 was increased at acidic pH in HT-29, HepG2, and BxPC3. HT-29 cells showed increased expressions of ERBB3, ATP6V0D2, and DMGDH at acidic pH, but the expressions of AREG and TNFRSF9 decreased. The expression of ATP6V0D2 was decreased by acidosis in BxPC3. The expressions of AREG, ERBB3, and ATP6V0D2 were not affected by pH in HepG2. These results suggested that genes whose expression is stimulated at acidic pH are different in different cells.
4. Discussion
The effect of acidosis on gene expression has been generally studied in medium without FBS until now. Cells are unable to proliferate under these conditions. In the present study, we used medium containing FBS which supported cell proliferation. We used medium without the addition of bicarbonate, because the medium pH was changed after the medium had been put into a CO2 incubator and the measurement of the exact pH value in the CO2 incubator was difficult. When the CO2 incubator is not used, the addition of bicarbonate is not necessary for cell proliferation. Bicarbonate is produced via metabolic processes, such as glycolysis and the citric acid cycle under aerobic conditions, and the production is enough for cell proliferation. In fact, all cell lines we used proliferated in medium without the addition of bicarbonate, and the proliferation rate was the same as that in medium with the addition of bicarbonate. The number of mesothelioma cells increased twofold during 2 days of incubation at pH 6.7 under our experimental conditions, but no proliferation was observed at pH 6.5 or less. We therefore used pH 6.7 medium under acidic conditions in this study.
Some diseased areas are acidified, but the acidification is less than 1 pH unit in many cases. Such a small change in pH has been thought to have little effect on mammalian cell functions until now. Our present data, however, clearly showed that acidification affects gene expression even if the pH change is small. Approximately 24,000 genes, about two-thirds of the mammalian genes, were analyzed in the present study, and 693 genes were up-regulated and 856 genes were down-regulated more than twofold at acidic pH in mesothelioma cells (Table 3, Table 6).
The expressions of 260 genes increased more than twofold in cells cultured at pH 6.7 for 2 h compared with pH 7.5. The expressions of 223 among the 260 genes decreased again after 24 h (Table 3). The physiological significance of the expression for a short time remains unclear. It is probably not due to the fluctuation of internal pH because the internal pH was decreased within 1 h after the acidic shift and then maintained at a constant level (data not shown). It has been generally accepted that the activation of the signal proteins increases rapidly after the stimulation and then decreases. It could be suggested that the expression levels of some genes for signal proteins decrease after the initial stimulation, although no direct evidence has yet been reported.
Our group found that the decrease in external pH below 7 changes the signal pathways, at least in part [11,12,15], and we identified a gene product that was essential for proliferation at acidic pH [13]. The present data showed that 84 genes for signaling were expressed more strongly after 24 h culture at acidic pH. The functions of the 78 genes whose expressions were up-regulated at acidic pH are unknown. It might be possible that some of these unidentified genes encode proteins for cellular signaling.
Since translational activities are different in different genes, the mRNA level is not proportional to the enzyme level. Therefore, all protein levels encoded by genes whose expression is affected by pH may be required for clarifying the signal pathways working at acidic pH. Furthermore, there are some genes whose expression is constitutive, but function is preferential at acidic pH. Lao et al. found CTIB to be essential for growth at acidic pH, but its expression was not affected by pH in the range from 6 to 8 [13,14,27]. p38 and ERK were activated strongly at acidic pH [12,15], but our present results showed no significant stimulation of their expression by acidosis. Identification of such proteins will be essential for improving our understanding of signal pathways operating under acidic diseased loci, and our present data could be useful for these studies as a database at the transcriptional level.
We found that different cytokines are expressed under different pH conditions (Table 4, Table 7). Especially IL-32 was found to express at a higher level at acidic pH in various cells. IL-32 was first identified in natural killer (NK) cells and IL-2 activated T cells [28], and was designated NK4. Since recombinant NK4 induced TNF-α production in human macrophages, it was assumed to have interleukin-like activity and hence was designated IL-32 [29]. Subsequent studies suggested that IL-32 is linked with pathological inflammation which often causes an acidic environment. Elevated IL-32 concentrations in synovial fluids and synovial tissues were demonstrated in rheumatoid arthritis but not in osteoarthritis patients [30,31]. Up-regulated IL-32 expression was also observed in the pancreatic ducts of chronic pancreatitis patients [32]. Taken together with our present data, IL-32 may be a factor that works under acidic conditions, but is not a cytokine specific to immune functions. IL-8, IL-15, and IL-16 were also up-regulated at acidic pH, and these interleukins may work in acidic diseased areas.
Our present data suggest that different signal pathways operate under different pH conditions. Why do mammalian cells have this multiplicity of signaling systems? The underlying mechanism is still unclear. Cytosolic pH changed with the change in extracellular pH, and the change in internal pH may affect protein activity because all proteins have pH-dependent activity. One possible explanation is that an enzyme having maximum activity at acidic pH works under acidic pH instead of the enzyme having maximum activity at alkaline pH. E. coli has multiple transport systems for sodium and potassium ions, and these systems work under different pH conditions [9,10]. Glycolysis was reported to increase in several tumors [1,2,3]. Only phosphoglycerate mutase 2 (muscle) was increased 2.03-fold at acidic pH (supplementary table), suggesting that other enzymes still work under acidic conditions without the elevation of transcription. Phosphoglycerate mutase 2 was reported to be a muscle-specific enzyme [33]. Since the muscles are often acidified, it can be argued that this enzyme works at acidic pH and the other isozyme does at alkaline pH.
The expressions of many receptor genes were affected by the pH change (Table 4, Table 7). Since receptors in the cytoplasmic membranes generally have a domain located outside the cells, the activity may be more sensitive to external acidosis compared with the cytosolic enzymes, and the gene expression of many receptors having an optimum activity at acidic pH may be stimulated by acidosis to compensate for the functional decline of receptors having an optimum activity at alkaline pH.
We used mesothelioma cells in the present study. Since the gene expression patterns were shown to be different in different cells, our present data may be applicable only to responses of mesothelioma cells. Analysis of the gene expressions in various cells, including non-tumor cells and normal tissues under acidic conditions will be essential for clarifying cell functions in acidic diseased areas.
5. Conclusions
Some diseased areas, such as cancer nests, inflammatory loci, and infarction areas, are acidified, but the acidification is less than 1 pH unit in many cases. Our present data clearly showed that acidification affects gene expression even if the pH change is small. Approximately 24,000 genes, about two-thirds of the mammalian genes, were analyzed using mesothelioma cells. The expressions of 693 genes were up-regulated more than twofold at acidic pH, and genes encoding proteins for signal pathways numbered 165 among the 693 genes. The expressions of 856 genes were down-regulated more than twofold at acidic pH, and 194 among the 856 genes encoded proteins for signal pathways.
Acknowledgements
We would like to express our thanks to K. Chiba (Graduate School of Pharmaceutical Sciences, Chiba University) for his gift of HepG2. This work was supported by Special Funds for Education and Research (Development of SPECT Probes for Pharmaceutical Innovation) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Supplementary Files
Supplemental Table (XLS, 12326 KB)
References
- 1.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 2.Helmlinger G., Yuan F., Dellian M., Jain R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Simmen H.P., Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am. J. Surg. 1993;166:24–27. doi: 10.1016/S0002-9610(05)80576-8. [DOI] [PubMed] [Google Scholar]
- 5.Goldie I., Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop. Scand. 1969;40:634–641. doi: 10.3109/17453676908989529. [DOI] [PubMed] [Google Scholar]
- 6.Ward T.T., Steigbigel R.T. Acidosis of synovial fluid correlates with synovial fluid leukocytosis. Am. J. Med. 1978;64:933–936. doi: 10.1016/0002-9343(78)90446-1. [DOI] [PubMed] [Google Scholar]
- 7.Geborek P., Saxne T., Pettersson H., Wollheim F.A. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J. Rheumatol. 1989;16:468–472. [PubMed] [Google Scholar]
- 8.Andersson S.E., Lexmüller K., Johansson A., Ekström G.M. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J. Rheumatol. 1999;26:2018–2024. [PubMed] [Google Scholar]
- 9.Ohyama T., Igarashi K., Kobayashi H. Physiological role of the chaA gene in sodium and calcium circulations at a high pH in Escherichia coli. J. Bacteriol. 1994;176:4311–4315. doi: 10.1128/jb.176.14.4311-4315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trchounian A., Kobayashi H. Kup is the major K+ uptake system in Escherichiacoli upon hyper-osmotic stress at a low pH. FEBS Lett. 1999;447:144–148. doi: 10.1016/S0014-5793(99)00288-4. [DOI] [PubMed] [Google Scholar]
- 11.Fukamachi T., Saito H., Kakegawa T., Kobayashi H. Different proteins are phosphorylated under acidic environments in Jurkat cells. Immunol. Lett. 2002;82:155–158. doi: 10.1016/S0165-2478(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 12.Hirata S., Fukamachi T., Sakano H., Tarora A., Saito H., Kobayashi H. Extracellular acidic environments induce phosphorylation of ZAP-70 in Jurkat T cells. Immunol. Lett. 2008;115:105–109. doi: 10.1016/j.imlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Lao Q., Fukamachi T., Saito H., Kuge O., Nishijima M., Kobayashi H. Requirement of an IκB-β COOH terminal region protein for acidic-adaptation in CHO cells. J. Cell Physiol. 2006;207:238–243. doi: 10.1002/jcp.20558. [DOI] [PubMed] [Google Scholar]
- 14.Fukamachi T., Lao Q., Okamura S., Saito H., Kobayashi H. CTIB (C-Terminus protein of IκB-β): a novel factor required for acidic adaptation. Adv. Exp. Med. Biol. 2006;584:219–228. doi: 10.1007/0-387-34132-3_16. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Hatatani K., Sun Y., Fukamachi T., Saito H., Kobayashi H. TCR signaling via ZAP-70 induced by CD3 stimulation is more active under acidic conditions. J. Cell Sci. Ther. 2012;S16:1. [Google Scholar]
- 16.Souza R.F., Shewmake K., Pearson S., Sarosi G.A., Jr., Feagins L.A., Ramirez R.D., Terada L.S., Spechler S.J. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G743–G748. doi: 10.1152/ajpgi.00144.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y., Lambert C.A., Colige A.C., Mineur P., Noël A., Frankenne F., Foidart J.M., Baba M., Hata R., Miyazaki K., et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J. Biol. Chem. 2005;280:10938–10944. doi: 10.1074/jbc.M411313200. [DOI] [PubMed] [Google Scholar]
- 18.Ihnatko R., Kubes M., Takacova M., Sedlakova O., Sedlak J., Pastorek J., Kopacek J., Pastorekova S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int. J. Oncol. 2006;29:1025–1033. [PubMed] [Google Scholar]
- 19.Xu L., Fukumura D., Jain R.K. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: Mechanism of low pH-induced VEGF. J. Biol. Chem. 2002;277:11368–11374. doi: 10.1074/jbc.M108347200. [DOI] [PubMed] [Google Scholar]
- 20.Elias A.P., Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron. 2008;1:131–139. doi: 10.1007/s12307-008-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjelmeland A.B., Wu Q., Heddleston J.M., Choudhary G.S., MacSwords J., Lathia J.D., McLendon R., Lindner D., Sloan A., Rich J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2010;18:829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X., Lucas J.E., Chen J.L., LaMonte G., Wu J., Wang M.C., Koumenis C., Chi J.T. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res. 2012;72:491–502. doi: 10.1158/0008-5472.CAN-11-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizarry R.A., Bolstad B.M., Collin F., Cope L.M., Hobbs B., Speed T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 25.Darnel J., Lodish H., Baltimore D. Molecular Cell Biology. Scientific American Books Inc.; New York, NY, USA: 1986. [Google Scholar]
- 26.Connor K.M., Hempel N., Nelson K.K., Dabiri G., Gamarra A., Belarmino J., van de Water L., Mian B.M., Melendez J.A. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007;67:10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 27.Lao Q., Kuge O., Fukamachi T., Kakegawa T., Saito H., Nishijima M., Kobayashi H. An IκB-β COOH terminal region protein is essential for the proliferation of CHO cells under acidic stress. J. Cell Physiol. 2005;203:186–192. doi: 10.1002/jcp.20221. [DOI] [PubMed] [Google Scholar]
- 28.Dahl C.A., Schall R.P., He H.L., Cairns J.S. Identification of a novel gene expressed in activated natural killer cells and T cells. J. Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 29.Kim S.H., Han S.Y., Azam T., Yoon D.Y., Dinarello C.A. Interleukin-32: A cytokine and inducer of TNFα. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Mun S.H., Kim J.W., Nah S.S., Ko N.Y., Lee J.H., Kim J.D., Kim D.K., Kim H.S., Choi J.D., Kim S.H., et al. Tumor necrosis factor α-induced interleukin-32 is positively regulated via the Syk/protein kinase Cδ/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 2009;60:678–685. doi: 10.1002/art.24299. [DOI] [PubMed] [Google Scholar]
- 31.Joosten L.A., Netea M.G., Kim S.H. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida A., Andoh A., Inatomi O., Fujiyama Y. Interleukin-32 expression in the pancreas. J. Biol. Chem. 2009;284:17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castella-Escola J., Ojcius D.M., LeBoulch P., Joulin V., Blouquit Y., Garel M.C., Valentin C., Rosa R., Climent-Romeo F., Cohen-Solal M. Isolation and characterization of the gene encoding the muscle-specific isozyme of human phosphoglycerate mutase. Gene. 1990;91:225–232. doi: 10.1016/0378-1119(90)90092-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table (XLS, 12326 KB)