Abstract
Background:
A number of preanalytical activities strongly influence sample quality, especially those related to sample collection. Since blood drawing through intravenous catheters is reported as a potential source of erythrocyte injury, we performed a critical review and meta-analysis about the risk of catheter-related hemolysis.
Materials and methods:
We performed a systematic search on PubMed, Web of Science and Scopus to estimate the risk of spurious hemolysis in blood samples collected from intravenous catheters. A meta-analysis with calculation of Odds ratio (OR) and Relative risk (RR) along with 95% Confidence interval (95% CI) was carried out using random effect mode.
Results:
Fifteen articles including 17 studies were finally selected. The total number of patients was 14,796 in 13 studies assessing catheter and evacuated tubes versus straight needle and evacuated tubes, and 1251 in 4 studies assessing catheter and evacuated tubes versus catheter and manual aspiration. A significant risk of hemolysis was found in studies assessing catheter and evacuated tubes versus straight needle and evacuated tubes (random effect OR 3.4; 95% CI = 2.9–3.9 and random effect RR 1.07; 95% CI = 1.06–1.08), as well as in studies assessing catheter and evacuated tubes versus catheter and manual aspiration of blood (OR 3.7; 95% CI = 2.7–5.1 and RR 1.32; 95% CI = 1.24–1.40).
Conclusions:
Sample collection through intravenous catheters is associated with significant higher risk of spurious hemolysis as compared with standard blood drawn by straight needle, and this risk is further amplified when intravenous catheter are associated with primary evacuated blood tubes as compared with manual aspiration.
Keywords: hemolysis, preanalytical variability, catheters, meta-analysis
Introduction
Preanalytical phase components are the leading causes of poor sample quality, wherein inappropriate or mishandled procedures for collecting blood specimens may be associated with a magnified risk of unsuitable samples (1,2). Among various preanalytical non-conformances that can be encountered in routine laboratory practice, sample hemolysis represents the primary source of problems, in terms of prevalence and likelihood of sample rejection (3,4). Spurious hemolysis, also referred to as “in vitro hemolysis”, is conventionally defined as erythrocyte injury or breakdown occurring during or after sample collection, once potential sources of hemolytic anemia have been ruled out. Although the observed frequency of spuriously hemolyzed samples varies widely throughout different healthcare settings, it can be estimated around ∼3% of all serum or plasma samples referred to central laboratories for routine or stat testing (5). It is also noteworthy that the vast majority of hemolyzed specimens come from the emergency department (ED), where the relative prevalence can be as high as 8–12% (6). The most frequent causes of spurious hemolysis include troublesome venipuncture(s), use of inappropriate blood collection devices, inappropriate handling (i.e., vigorous mixing) and transportation (i.e., freezing or trauma) of blood tubes (4,7). Regardless of specific causes, the receipt of hemolyzed specimens is always a problem, wherein test results of some analytes such as potassium, lactate dehydrogenase (LD), aspartate aminotransferase (AST) or cardiospecific troponins among others should be suppressed, reported with comments, corrected or recalculated or even provided with semi-quantative comments indicating likely range of results (8,9). This obviously causes diagnostic delays, incremental costs for material used for redrawing blood, as well as interrelational problems with hospital physicians and nurses (6).
Among potential sources of erythrocyte injury, blood drawing through intravenous catheters has been reported as an important factor in a variety of studies and reviews, which however provided rather different estimations of frequency, nor have these studies exactly quantified the risk of catheter-related hemolysis (10,11). In this study we performed a systematic search of current scientific literature, to estimate the cumulative risk of spurious hemolysis in samples collected from intravenous catheters.
Materials and methods
Search methodology
A systematic electronic search was performed on the three most frequently used scientific databases (i.e., PubMed, Web of Science and Scopus) (12), with no date restriction, to retrieve all published studies up to February 2013 that investigated the rate of hemolysis in blood drawn by intravenous catheters, either comparing intravenous catheter collection combined with evacuated blood tubes versus straight needle collection combined with evacuated blood tubes, or intravenous catheter collection combined with evacuated blood tubes versus intravenous catheter collection combined with manual aspiration of blood. The following keywords were used: “hemolysis” or “haemolysis”, in combination with “catheter” or “intravenous line”. The bibliographic references of items published in English, French, Spanish and Italian were reviewed for additional relevant studies. All the articles identified according to these search criteria were systematically assessed for quality by two authors (GL and CM), according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist criteria (13). Disagreements were solved by a third opinion (GC).
Statistical analysis
Heterogeneity was assessed by chi-square based statistics and I-square test, whereas the publication bias was evaluated using Egger test. A sensitivity analysis was performed according to publication as full-length article. The Odds ratio (OR), Relative risk (RR) and 95% Confidence interval (95% CI) were calculated using a random effect model, as for I-square values greater than 50%. Statistical analysis was performed with MedCalc Version 12.3.0 (MedCalc Software, Mariakerke, Belgium).
Results
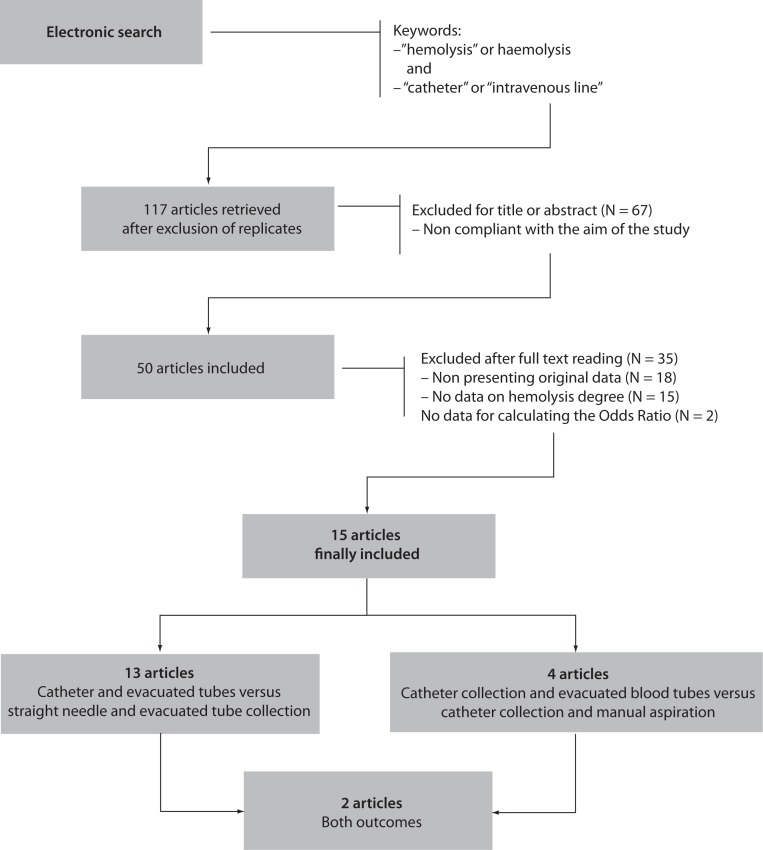
The electronic search according to the above mentioned criteria identified 117 citations of articles and abstracts after elimination of replicates among the searchable databases (Figure 1). Sixty seven items were immediately excluded after title and/or abstract consultation, because not compliant with the aim of this study (i.e., most of these were microbiological studies assessing the hemolysis potential of bacteria and other microorganisms). Eighteen items were excluded after full text reading because not showing original data (i.e., editorials or critical reviews of the literature), whereas 15 articles were excluded because they did not contain specific data about hemolysis. The remaining 17 items were carefully assessed for quality after revision of the text, and two were excluded because they did not contain sufficient information for calculating OR and RR. Inter-rater agreement was excellent (98%; kappa statistic = 0.88; P < 0.001).
Figure 1.
Prospect of electronic search and study evaluation.
Overall, 15 articles were finally selected for inclusion in meta-analysis (14–28). Eleven of these articles showed data on catheter combined with evacuated tubes collection versus straight needle combined with evacuated tubes collection, two on catheter combined with evacuated blood tubes collection versus catheter combined with manual aspiration of blood, and two on both study outcomes (Figure 1). Therefore, the final number of studies assessing catheter combined with evacuated tubes collection versus straight needle combined with evacuated tubes collection was 13, whereas those comparing catheter combined with evacuated blood tubes collection versus catheter combined with manual aspiration were 4, for a total of 17 studies in the 15 articles included. The total number of patients was 14,796 in 13 studies assessing catheter combined with evacuated tubes versus straight needle combined with evacuated tubes, and 1251 in 4 studies assessing catheter combined with evacuated tubes versus catheter combined with manual blood aspiration. The inter-study variation was high and attributable to heterogeneity for sample size (chi-squared, 3185; DF, 17; I-squared, 99.8%; P < 0.001), modality of sample hemolysis assessment (chi-squared, 1699; DF, 17; I-squared, 99.0%; P < 0.001), as well as for hemolysis threshold (chi-squared, 1172; DF, 17; I-squared, 98.6%; P < 0.001). All studies were based in EDs. No publication bias was found by Egger regression test (P = 0.68).
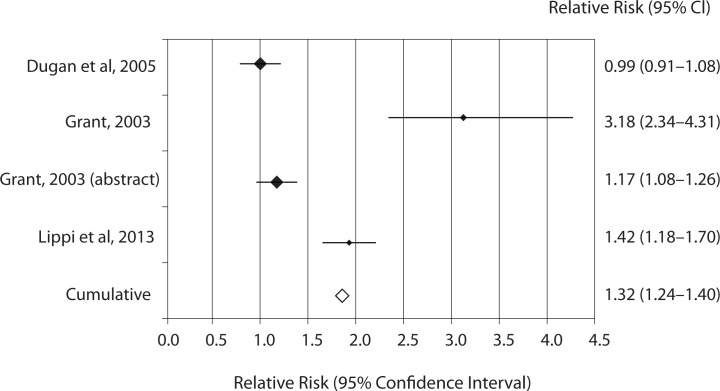
The results of individual studies along with random effect ORs and RRs for catheter-related hemolysis are shown in table 1 and 2. The range of individual ORs (3.4–116.7) and RRs (1.01–4.31) were statistically significant in 11/13 studies comparing catheter and evacuated tubes with straight needle and evacuated tubes, whereas the range of individual ORs (0.9–22.7) and RRs (0.99–3.18) were statistically significant in 3/4 studies comparing catheter and evacuated tubes with catheter and manual blood aspiration. Significant risk of spurious hemolysis was found pooling results of 13 individual studies assessing catheter and evacuated tubes versus straight needle and evacuated tubes, showing random effect OR of 3.4 (95% CI = 2.9–3.9; P < 0.001) and RR of 1.07 (95% CI = 1.06–1.08; P < 0.001) (Table 1). The sensitivity analysis did not reveal a significant impact of publication status on these results after excluding trials that were not published as full-length articles (OR 3.1 versus 3.4; RR 1.07 for both cases). Similarly, a statistically significant risk was observed pooling results of 4 individual studies assessing catheter and evacuated tubes versus catheter and manual blood aspiration blood, showing random effect OR of 3.7 (95% CI = 2.7–5.1; P < 0.001) and RR of 1.32 (95% CI = 1.24–1.40; P < 0.001) (Table 2 and Figure 2). The sensitivity analysis did not reveal a significant impact of publication status on these results after excluding the trial that was not published as full-length article (OR 3.6 versus 3.7; RR 1.40 versus 1.32). No study directly compared catheter and manual aspiration collection versus straight needle and evacuated tube.
Table 1.
Individual studies and random effect Odds ratio (OR) and Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus straight needle combined with evacuated tube collection in the emergency department.
| Study | Patients | No catheter | Catheter | OR | RR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Authors | Hemolysis assessment | Hemolysis threshold | Total | No hemolysis | Hemolysis | No hemolysis | Hemolysis | Value | 95% CI | P | Value | 95% CI | P |
| Agós et al. 2008 | Visual inspection | NA | 1933 | 341 | 7 | 1363 | 222 | 7.9 | 3.7–17.0 | < 0.001 | 1.14 | 1.11–1.17 | < 0.001 |
| Burns et al. 2002 | Visual inspection | NA | 200 | 37 | 0 | 141 | 22 | 11.9 | 0.7–201.1 | 0.085 | 1.14 | 1.07–1.23 | < 0.001 |
| Dietrich, 2013 | Hemolysis Index | 2.0 g/L | 7104 | 3298 | 3 | 3762 | 41 | 12.0 | 3.7–38.7 | < 0.001 | 1.01 | 1.01–1.01 | < 0.001 |
| Fang et al. 2008 | Visual inspection | NA | 156 | 108 | 32 | 8 | 8 | 3.4 | 1.2–9.7 | 0.024 | 1.54 | 0.94–2.54 | 0.088 |
| Giavarina et al. 2010 | Hemolysis Index | 0.8 g/L | 421 | 97 | 3 | 257 | 64 | 8.1 | 2.5–26.2 | < 0.001 | 1.21 | 1.14–1.29 | < 0.001 |
| Grant, 2003 | Visual inspection | NA | 300 | 102 | 3 | 44 | 151 | 116.7 | 35.3–385.9 | < 0.001 | 4.31 | 3.31–5.60 | <0.001 |
| Grant, 2003 (abstract) | Visual inspection | NA | 372 | 116 | 1 | 204 | 51 | 29.0 | 4.0–212.6 | < 0.001 | 1.24 | 1.16–1.32 | < 0.001 |
| Kennedy et al. 2006 | Visual inspection | NA | 165 | 75 | 3 | 75 | 12 | 4.0 | 1.1–14.8 | 0.037 | 1.12 | 1.01–1.23 | 0.024 |
| Lowe et al. 2008 | Visual inspection | 0.5 g/L | 853 | 354 | 1 | 470 | 28 | 21.1 | 2.9–155.7 | 0.003 | 1.06 | 1.03–1.08 | < 0.001 |
| Munnix et al. 2011 | Hemolysis Index | 0.3 g/L | 150 | 50 | 0 | 84 | 16 | 19.7 | 1.2–335.9 | 0.039 | 1.18 | 1.08–1.30 | < 0.001 |
| Ong et al. 2008 | Visual inspection | NA | 227 | 55 | 4 | 127 | 41 | 4.4 | 1.5–13.0 | 0.006 | 1.23 | 1.10–1.38 | < 0.001 |
| Straszewski et al. 2011 | Visual inspection | NA | 2879 | 2395 | 169 | 243 | 72 | 4.2 | 3.1–5.7 | < 0.001 | 1.21 | 1.14–1.29 | < 0.001 |
| Seemann et al. 2000 | Visual inspection | NA | 36 | 19 | 0 | 13 | 4 | 13.0 | 0.6–261.9 | 0.094 | 1.30 | 0.99–1.71 | 0.062 |
|
| |||||||||||||
| Total | 14796 | 7047 | 226 | 6791 | 732 | 3.4 | 2.9–3.9 | < 0.001 | 1.07 | 1.06–1.08 | < 0.001 | ||
ED - emergency department; 95% CI - 95% confidence interval; NA – not available.
Table 2.
Individual studies and random effect Odds ratio (OR) and Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus catheter combined with manual aspiration collection in the emergency department.
| Study | Patients | Syringe | Evacuated tube | OR | RR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Authors | Hemolysis assessment | Hemolysis threshold | Total | No hemolysis | Hemolysis | No hemolysis | Hemolysis | Value | 95% CI | P | Value | 95% CI | P |
| Lippi et al. 2013 | Hemolysis Index | 0.5 g/L | 104 | 51 | 1 | 36 | 16 | 22.7 | 2.9–178.7 | 0.003 | 1.42 | 1.18–1.70 | < 0.001 |
| Dugan et al. 2005 | Visual inspection | 2.0 g/L | 382 | 90 | 14 | 243 | 35 | 0.9 | 0.5–1.8 | 0.82 | 0.99 | 0.91–1.08 | 0.82 |
| Grant, 2003 | Visual inspection | NA | 255 | 43 | 17 | 44 | 151 | 8.7 | 4.5–16.7 | < 0.001 | 3.18 | 2.34–4.31 | < 0.001 |
| Grant, 2003 (abstract) | Visual inspection | NA | 510 | 232 | 23 | 199 | 56 | 2.8 | 1.7–4.8 | < 0.001 | 1.17 | 1.08–1.26 | < 0.001 |
|
| |||||||||||||
| Total | 1251 | 416 | 55 | 522 | 258 | 3.7 | 2.7–5.1 | < 0.001 | 1.32 | 1.24–1.40 | < 0.001 | ||
ED - emergency department; 95% CI - 95% confidence interval; NA – not available.
Figure 2.
Individual studies and random effect Relative risk (RR) of spurious hemolysis in catheter combined with evacuated tubes collection versus catheter combined with manual aspiration collection.
Discussion
The results of this meta-analysis - which is limited to published data and ED setting - attest that sample collection through intravenous catheters is associated with significantly higher risk (i.e., 7%) of spurious hemolysis as compared with standard blood drawn by straight needles, and that this risk is further amplified when intravenous catheter are associated with primary evacuated blood tubes as compared with manual blood aspiration with syringes or S-Monovette® blood tubes (Sarstedt AG & Co., Nümbrecht, Germany) used in manual aspiration mode (29). According to these findings, it seems reasonable to conclude that blood drawing by straight needle venipuncture should be preferred over intravenous catheter collection for reducing the chance of erythrocyte injury, although this approach may appear impractical in health-care setting such as ED or Intensive care units (ICU) where intravenous lines are systematically placed upon patient admission. A potential option to reduce the chance of collecting unsuitable samples entails the use of manual aspiration rather than vacuum force for drawing blood from intravenous catheters (Figure 2), since the former practice causes a larger shear stress due to the collapse of soft plastic material under negative pressure when blood is aspirated by the vacuum, as well as turbulence due to difference of pressures between veins, catheter needles, valves and evacuated collection tubes (11).
The large heterogeneity in methods used to identify unsuitable samples (i.e., hemolysis index versus visual inspection), which is partially attributable to the fact that automatic performance of serum indices has only recently become available in laboratory instrumentation (30), is another important finding, which calls for additional practical considerations. First, visual inspection is arbitrary, cannot be standardized and is characterized by small inter-observer agreement (31), so that it should be replaced by automated systems that report serum indices. The hemolysis index is in fact accurate when compared with the reference cyanmethemoglobin assay, and is also highly reproducible among different platforms and laboratories (31,32). This aspect becomes crucial considering that increasing implementation of continuous-flow automation in clinical laboratory abolishes the chance of visually inspecting the samples, thus making automatic performance of serum indices virtually unavoidable (31,33). Inherently linked to this point is the lack of consensus about hemolysis thresholds across studies where the cut-off of cell-free hemoglobin has been reported (ranging from 0.3 up to 2.0 g/L). Besides Italian national recommendations, concluding that a sample should be considered “modestly” or “frankly” hemolyzed when cell-free hemoglobin concentration is 0.3–0.6 g/L and > 0.6 g/L, respectively (34), there are no other guidelines about this issue. This is a gap that the new European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group on preanalytical variability is supposed to close, by issuing official recommendations for harmonizing policies for rejecting hemolyzed samples throughout Europe (35).
Footnotes
Potential conflict of interest
None declared.
References
- 1.Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49:1113–26. doi: 10.1515/CCLM.2011.600. http://dx.doi.org/10.1515/cclm.2011.600. [DOI] [PubMed] [Google Scholar]
- 2.Simundic AM, Lippi G. Preanalytical phase--a continuous challenge for laboratory professionals. Biochem Med. 2012;22:145–9. doi: 10.11613/bm.2012.017. http://dx.doi.org/10.11613/BM.2012.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost. 2012;38:565–75. doi: 10.1055/s-0032-1315961. http://dx.doi.org/10.1055/s-0032-1315961. [DOI] [PubMed] [Google Scholar]
- 4.Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolysed specimens. Biochem Med. 2010;20:154–9. http://dx.doi.org/10.11613/BM.2010.018. [Google Scholar]
- 5.Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000;46:306–7. [PubMed] [Google Scholar]
- 6.Lippi G, Plebani M, Di Somma S, Cervellin G. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci. 2011;48:143–53. doi: 10.3109/10408363.2011.600228. http://dx.doi.org/10.3109/10408363.2011.600228. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46:764–72. doi: 10.1515/CCLM.2008.170. http://dx.doi.org/10.1515/CCLM.2008.170. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Avanzini P, Pavesi F, Bardi M, Ippolito L, Aloe R, Favaloro EJ. Studies on in vitro hemolysis and utility of corrective formulas for reporting results on hemolyzed specimens. Biochem Med. 2011;21:297–305. doi: 10.11613/bm.2011.040. http://dx.doi.org/10.11613/BM.2011.040. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Avanzini P, Dipalo M, Aloe R, Cervellin G. Influence of hemolysis on troponin testing: studies on Beckman Coulter UniCel Dxl 800 Accu-TnI and overview of the literature. Clin Chem Lab Med. 2011;49:2097–100. doi: 10.1515/CCLM.2011.703. http://dx.doi.org/10.1515/CCLM.2011.703. [DOI] [PubMed] [Google Scholar]
- 10.Heyer NJ, Derzon JH, Winges L, Shaw C, Mass D, Snyder SR, et al. Effectiveness of practices to reduce blood sample hemolysis in EDs: a laboratory medicine best practices systematic review and meta-analysis. Clin Biochem. 2012;45:1012–32. doi: 10.1016/j.clinbiochem.2012.08.002. http://dx.doi.org/10.1016/j.clinbiochem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halm MA, Gleaves M. Obtaining blood samples from peripheral intravenous catheters: best practice? Am J Crit Care. 2009;18:474–8. doi: 10.4037/ajcc2009686. http://dx.doi.org/10.4037/ajcc2009686. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Favalor EJ, Simundic AM. Biomedical research platforms and their influence on article submissions and journal rankings: an update. Biochem Med. 2012;22:7–14. http://dx.doi.org/10.11613/BM.2012.002. [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. http://dx.doi.org/10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agós MD, Lizarraga R, Gambra D, Marañón A, Orozco C, Díaz E. Factors related to haemolysis in the extraction of blood samples. An Sist Sanit Navar. 2008;31:153–8. doi: 10.4321/s1137-66272008000300005. [DOI] [PubMed] [Google Scholar]
- 15.Burns ER. Hemolysis in serum samples drawn by emergency department personnel versus laboratory phlebotomists. Lab Medicine. 2002;33:378–80. http://dx.doi.org/10.1309/PGM4-4F8L-2P1M-LKPB. [Google Scholar]
- 16.Dietrich H. One Poke or Two: Can Intravenous Catheters Provide an Acceptable Blood Sample? A Data Set Presentation, Review of Previous Data Sets, and Discussion. J Emerg Nurs. 2013 doi: 10.1016/j.jen.2012.11.002. [Epub ahead of print] http://dx.doi.org/10.1016/j.jen.2012.11.002. [DOI] [PubMed]
- 17.Fang L, Fang SH, Chung YH, Chien ST. Collecting factors related to the haemolysis of blood specimens. J Clin Nurs. 2008;17:2343–51. doi: 10.1111/j.1365-2702.2006.02057.x. http://dx.doi.org/10.1111/j.1365-2702.2006.02057.x. [DOI] [PubMed] [Google Scholar]
- 18.Giavarina D, Pasquale L, Mezzena G, Soffiati G. Hemolysis by peripheral intravenous catheters: materials comparison. RIMeL/IJLaM. 2010;6:216–21. [Google Scholar]
- 19.Grant MS. The effect of blood drawing techniques and equipment on the hemolysis of ED laboratory blood samples. J Emerg Nurs. 2003;29:116–21. doi: 10.1067/men.2003.66. http://dx.doi.org/10.1067/men.2003.66. [DOI] [PubMed] [Google Scholar]
- 20.Grant MS. The effect of blood drawing techniques and equipment on the hemolysis of ED laboratory blood samples [Abstract] J Emerg Nurs. 2003;29:406. doi: 10.1067/men.2003.66. http://dx.doi.org/10.1016/S0099-1767(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy C, Angermuller S, King R, Noviello S, Walker J, Warden J, Vang S. A comparison of hemolysis rates using intravenous catheters versus venipuncture tubes for obtaining blood samples. J Emerg Nurs. 1996;22:566–9. doi: 10.1016/s0099-1767(96)80213-3. http://dx.doi.org/10.1016/S0099-1767(96)80213-3. [DOI] [PubMed] [Google Scholar]
- 22.Lowe G, Stike R, Pollack M, Bosley J, O’Brien P, Hake A, et al. Nursing blood specimen collection techniques and hemolysis rates in an emergency department: analysis of veni-puncture versus intravenous catheter collection techniques. J Emerg Nurs. 2008;34:26–32. doi: 10.1016/j.jen.2007.02.006. http://dx.doi.org/10.1016/j.jen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Munnix IC, Schellart M, Gorissen C, Kleinveld HA. Factors reducing hemolysis rates in blood samples from the emergency department. Clin Chem Lab Med. 2011;49:157–8. doi: 10.1515/CCLM.2011.012. http://dx.doi.org/10.1515/cclm.2011.012. [DOI] [PubMed] [Google Scholar]
- 24.Ong ME, Chan YH, Lim CS. Observational study to determine factors associated with blood sample haemolysis in the emergency department. Ann Acad Med Singapore. 2008;37:745–8. [PubMed] [Google Scholar]
- 25.Straszewski SM, Sanchez L, McGillicuddy D, Boyd K, Dufresne J, Joyce N, et al. Use of separate venipunctures for IV access and laboratory studies decreases hemolysis rates. Intern Emerg Med. 2011;6:357–9. doi: 10.1007/s11739-011-0568-9. http://dx.doi.org/10.1007/s11739-011-0568-9. [DOI] [PubMed] [Google Scholar]
- 26.Seemann S, Reinhardt A. Blood sample collection from a peripheral catheter system compared with phlebotomy. J Intraven Nurs. 2000;23:290–7. [PubMed] [Google Scholar]
- 27.Lippi G, Avanzini P, Cervellin G. Prevention of hemolysis in blood samples collected from intravenous catheters. Clin Biochem. 2013 doi: 10.1016/j.clinbiochem.2013.01.021. [Epub ahead of print] http://dx.doi.org/10.1016/j.clinbiochem.2013.01.021. [DOI] [PubMed]
- 28.Dugan L, Leech L, Speroni KG, Corriher J. Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs. 2005;31:338–45. doi: 10.1016/j.jen.2005.05.004. http://dx.doi.org/10.1016/j.jen.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Lippi G, Avanzini P, Musa R, Sandei F, Aloe R, Cervellin G. Evaluation of sample hemolysis in blood collected by S-Monovette® using vacuum or aspiration mode. Biochem Med. 2013;23:64–9. doi: 10.11613/BM.2013.008. http://dx.doi.org/10.11613/BM.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plebani M, Lippi G. Hemolysis index: quality indicator or criterion for sample rejection? Clin Chem Lab Med. 2009;47:899–902. doi: 10.1515/CCLM.2009.229. http://dx.doi.org/10.1515/CCLM.2009.229. [DOI] [PubMed] [Google Scholar]
- 31.Simundic AM, Nikolac N, Ivankovic V, Ferenec-Ruzic D, Magdic B, Kvaternik M, Topic E. Comparison of visual vs. automated detection of lipemic, icteric and hemolyzed specimens: can we rely on a human eye? Clin Chem Lab Med. 2009;47:1361–5. doi: 10.1515/CCLM.2009.306. http://dx.doi.org/10.1515/CCLM.2009.306. [DOI] [PubMed] [Google Scholar]
- 32.Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med. 2009;47:934–9. doi: 10.1515/CCLM.2009.218. http://dx.doi.org/10.1515/CCLM.2009.218. [DOI] [PubMed] [Google Scholar]
- 33.Lippi G, Plebani M. Continuous-Flow Automation and Hemolysis Index: A Crucial Combination. J Lab Autom. 2012 doi: 10.1177/2211068212450014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Caputo M, Banfi G, Daves M, Dolci A, Montagnana M, et al. SIBioC-SIMeL consensus recommendations for the identification and management of hemolysed specimens and the implementation of hemolysis index. Biochim Clin. 2011;35:481–90. [Google Scholar]
- 35.European Federation of Clinical Chemistry and Laboratory Medicine Working Group on Preanalytical Phase. Available at: http://efcclm.eu/science/wg-preanalytical-phase.: Accessed 13 February 2013.