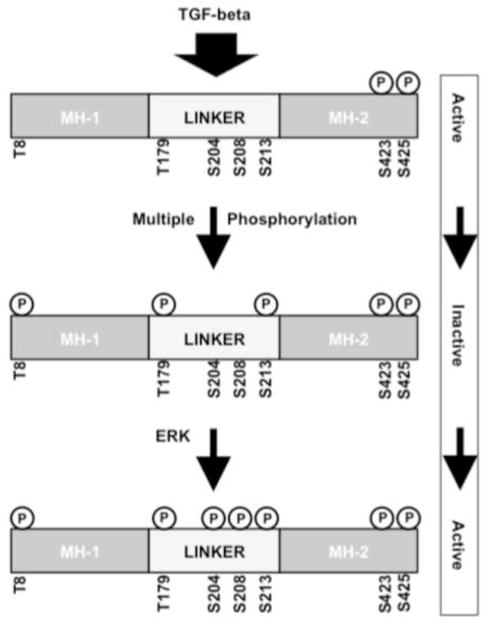
Figure 6. Proposed model for regulation of Smad3 activity in TGF-β-stimulated collagen expression.
TGF-β stimulates the TβR to phosphorylate essential serines at the carboxyl terminus of Smad3. In the absence of linker region phosphorylation, Smad3 can activate the COL1A2 promoter. Phosphorylation of T179 and S213 is likely to be inhibitory, whereas ERK-mediated phosphorylation at S204 and possibly S208 enhances responses, likely by releasing the inhibition caused by phosphorylation at the T179 and S213 sites. Since ERK inhibition targets S204 phosphorylation, we propose that S204 phosphorylation is an important, ERK-mediated step in TGF-β–stimulated collagen expression. This complex model could explain both our results and the often-contradictory findings reported in the literature.