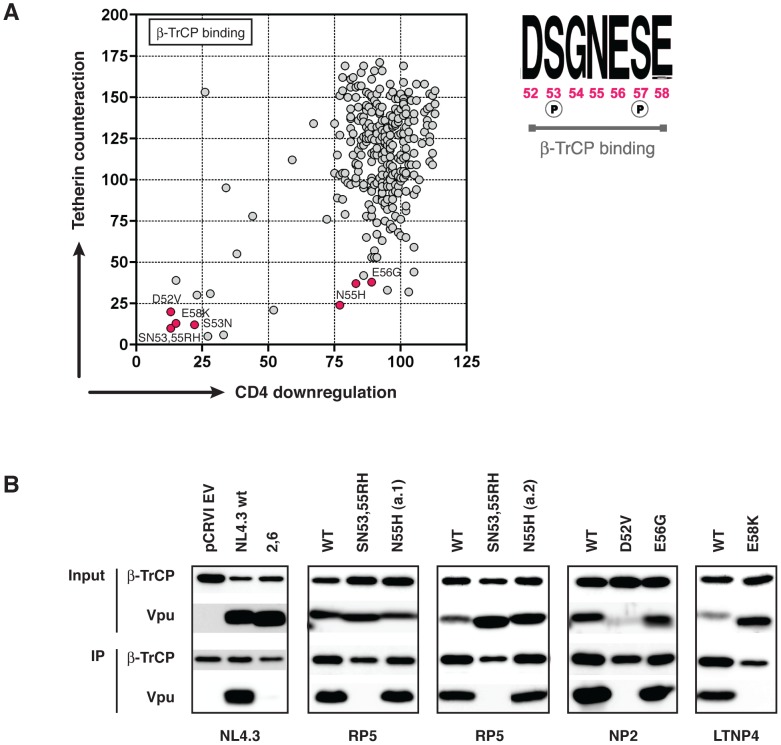
Figure 5. Further investigation of the β-TrCP binding properties of the N55H and E56G Vpu mutants.
(A) We identified seven naturally defective Vpus with mutations in the DSGNES β-TrCP binding site, or in the adjacent glutamic acid that is essential for the casein kinase II-mediated phosphorylation of serines 53 and 57. Six of these mutants were tested for their ability to bind β-TrCP in co-immunoprecipitation experiments (B). Vpu negative (pCRVI), NL4.3 wt (positive control) and NL4.3 S52,56A mutant (negative control) are shown for comparison. In all cases, the mutant Vpu was compared to its closest functional relative (wt). Vpus are therefore described as the wt Vpu from that individual, plus the position and type of the DSGNES mutation. 293T cells were co-transfected with pCRVI-Vpu (or EV) and pCR3.1-myc β-TrCP (or GFP) and 48 hours later lysed and immunoprecipitated with anti-myc antibody.