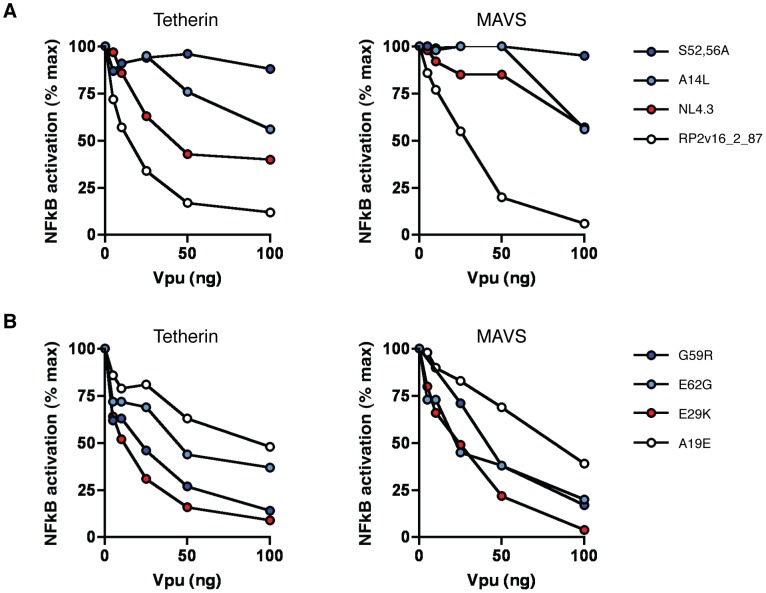
Figure 8. Ability of Vpu to suppress NF-κB activation.
(A) NL4.3 Vpu, NL4.3 A14L and S52,56A mutants, and the highly active patient-derived Vpu RP2v16_2_87 were tested at a range of concentrations for their ability to counteract tetherin-mediated NF-κB activation (left panel) and MAVS-mediated NF-κB activation (right panel). Transient NF-κB activation assays were performed by transfecting 293 cells with 50 ng pCR3.1 human tetherin or 10 ng pCR3.1 MAVS alongside 0, 5, 10, 25, 50 and 100 ng of each pCRVI-Vpu. Fold activation of a luciferase NF-κB reporter gene is calculated relative to a GFP control in the presence of increasing concentrations of Vpu, and results are presented relative to the mean signal obtained in the absence of Vpu (% max). (B) As for (A), but with patient-derived Vpus with defects in tetherin signalling suppression but not promotion of virus release (G59R, E62G); patient-derived Vpu with defects in promotion of virus release but not suppression of signalling (E29K); and patient-derived Vpu with defects in both tetherin signalling suppression and promotion of virus release (A19), all identified in Figure 7 .