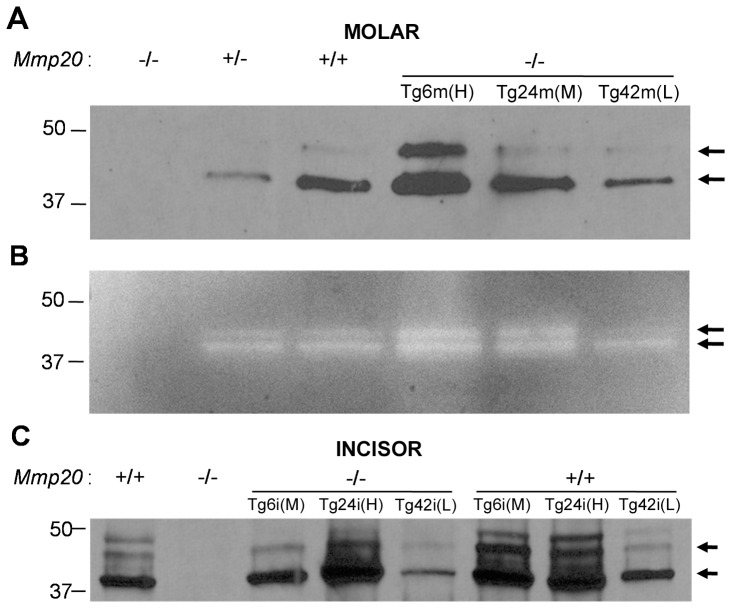
Figure 2. Assessment of MMP20 protein content from extracted enamel.
Immunoblots performed on proteins extracted from 5 day-old molars (A) or adult incisors (C) assessed MMP20 quantity. Zymography of extracted enamel from 5 day-old molars assessed MMP20 proteolytic activity (B). MMP20 was not detected in Mmp20 null (–/–) mouse enamel, low levels were observed in the heterozygous (+/–) enamel and wild-type (+/+) enamel had more MMP20 protein than did the heterozygotes. In molar (m) enamel from Mmp20 null mice, the Tg6 transgene [Tg6m (H)] expressed the highest quantities of MMP20 followed by the Tg24m (M) transgene at mid-levels with the Tg42m (L) transgene expressing the lowest levels of MMP20. The Tg42m (L) transgene expressed lower MMP20 amounts than were present in enamel from wild-type mice (A). Zymography results for MMP20 activity (B) were consistent with the immunoblot results. In contrast to the molar results, immunoblots performed on extracted incisor (i) enamel showed that enamel from Tg24i (H) transgenic mice contained the highest amount of MMP20 protein followed by Tg6i (M) enamel and then Tg42i (L) transgenic enamel which, like the molars, also contained less MMP20 than wild-type enamel (C). As expected, enamel from transgenes expressed in the Mmp20 wild-type background had total MMP20 quantities that were higher for each transgene than were observed in the null background. Arrows point to the MMP20 doublet bands that each represents an active form of MMP20.