Figure 2.
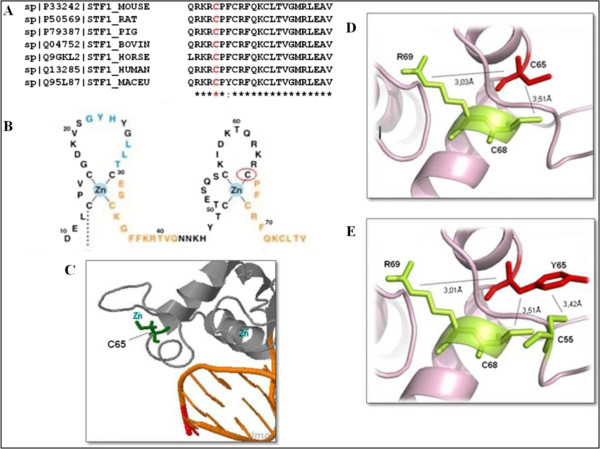
Comparison of normal and mutant NR5A1 protein considering sequence conservation and internal aminoacid interactions. A) Multiple alignment of NR5A1 protein family using ClustalW: the conserved residue C65 is shown in red. B) Scheme of the two zinc fingers from the DNA-binding domain (DBD). Red circle denotes the C65 residue (adapted from Little et al. [25]). C) Structural complex of NR5A1 bound to DNA showing the C65 residue ligated to the zinc atom at the zinc-finger binding site within the DNA binding domain. D) Structural model of the native protein showing internal contacts. The C65 interacts by hydrogen bond with R69 and hydrophobic interaction with C68. E) Mutant protein internal contacts. The Y65 establish new hydrophobic contact with C55.
