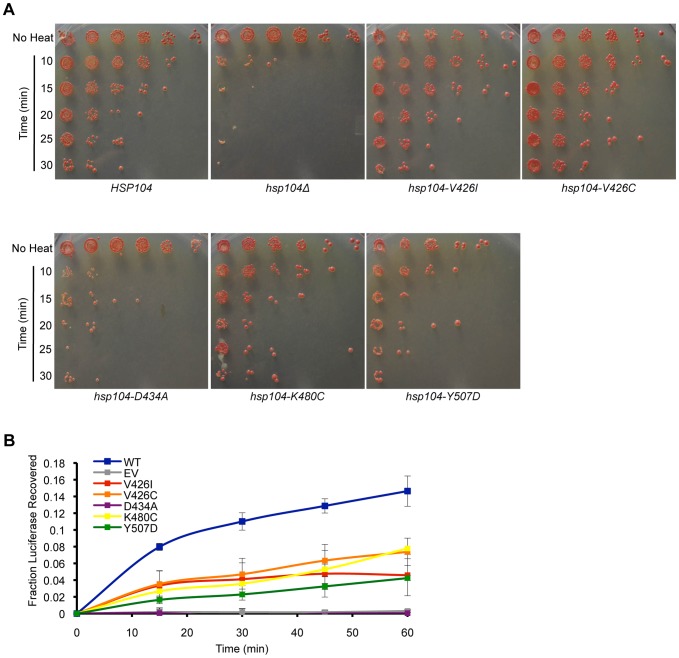
Figure 5. M-domain mutants have differing effects on the ability to disaggregate non-prion substrates.
(A) hsp104Δ strains expressing wild type HSP104, hsp104-V426I, hsp104-V426C, hsp104-D434A, hsp104-K480C, hsp104-Y507D from a HIS3-containing plasmid, or an empty vector control (hsp104Δ), were heat shocked to measure the mutants' ability to confer thermotolerance. Cultures were grown at 37°C to induce Hsp104 expression, then heat shocked at 50°C for various amounts of time, as compared to controls with no heat shock (No Heat), serially diluted five-fold, and spotted on medium lacking histidine to assess viability. Data are representative of three individual experiments. (B) hsp104Δ strains containing a plasmid expressing luciferase and expressing wild type (WT) HSP104 (blue), hsp104-V426I (red), hsp104-V426C (orange), hsp104-D434A (purple), hsp104-K480C (yellow), hsp104-Y507D (green), or an empty vector (EV) control (gray) were grown at 37°C to induce Hsp104 expression, then heat shocked at 44°C for an hour to induce luciferase aggregation. At the indicated times during recovery at 30°C, samples were taken, luciferin was added, and the luminescence was measured. The graph represents the amount of luciferase recovered as a fraction of the total luciferase before heat shock. Three separate samples for each mutant were analyzed and error bars reflect standard deviation between the samples.