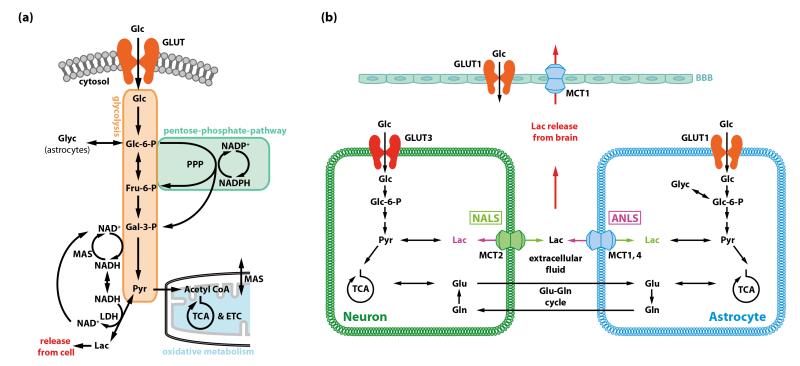
Figure 2. Generation of energy in brain and three models for the fate of lactate derived from glucose metabolism in the brain.
(a) Major pathways of glucose metabolism. Hexokinase uses ATP to phosphorylate glucose to glucose-6-phosphate (Glc-6-P) in the first irreversible step of the glycolytic pathway. Glc-6-P regulates hexokinase activity by feedback inhibition [19], and it is a ‘branch-point’ metabolite that has alternative metabolic fates. Glc-6-P can continue down the glycolytic pathway to generate pyruvate that can then be used in mitochondria by oxidative metabolism via the tricarboxylic acid (TCA) cycle. It can also enter the pentose phosphate shunt pathway (PPP) to generate NADPH for management of oxidative stress and precursors for nucleic acid biosynthesis, and, in astrocytes, it is a precursor for glycogen. Most of the glucose carbon derived from the PPP re-enters the glycolytic pathway downstream of Glc-6-P. The glycolytic pathway produces a net of 2 ATP per molecule of glucose and oxidation of pyruvate via acetyl coenzyme A (acetyl CoA) in the TCA cycle produces about 30 ATP for a total of about 32 ATP. Formation of pyruvate from glucose requires regeneration of NAD+ from NADH produced by the glyceraldehyde-3-phosphate dehydrogenase reaction by the malateaspartate shuttle (MAS). NADH cannot cross the mitochondrial membrane, and the MAS transfers cytoplasmic NADH to the mitochondria where it is oxidized via the electron transport chain (ETC). When glycolytic flux exceeds that of the MAS or the TCA cycle rate, or during hypoxic or anoxic conditions, NAD+ is regenerated by the lactate dehydrogenase (LDH) reaction that converts pyruvate to lactate. Because intracellular accumulation of lactate would cause reversal of the LDH reaction, lactate must be released from the cell by monocarboxylic acid transporters (MCT). Exit of lactate eliminates pyruvate as an oxidizable substrate for that cell and limits the ATP yield per glucose to two. (b) Three models for the fate of lactate generated in brain from blood-borne glucose or astrocytic glycogen. The astrocyte-to-neuron lactate shuttle (ANLS) was proposed on the basis of glutamate-evoked increases in glucose utilization and lactate release by cultured astrocytes (reviewed in [29]). In brief, the model states that Na+-dependent uptake of neurotransmitter glutamate from the synaptic cleft by astrocytes generates a demand for 2 ATP in astrocytes, one to extrude Na+ and one to convert glutamate into glutamine in the glutamate-glutamine cycle (Glossary). The model states that this ATP is generated by the glycolytic pathway and is associated with release of lactate from astrocytes and its uptake by nearby neurons where it is oxidized. Thereby astrocyte-neuron metabolic coupling is linked with the glutamate-glutamine cycle and excitatory neurotransmission. Thus, during brain activation glycolytic upregulation is stated to occur in astrocytes, with astrocyte-derived lactate providing the major fuel for neurons. The neuron-toastrocyte lactate shuttle (NALS) is based on kinetics of glucose uptake into brain cells in response to increased metabolic demand and different model assumptions compared with the ANLS [27]. Here, glucose is predicted to be predominantly taken up into neurons due to their high energy demand and the higher transport rate of the neuronal glucose transporter, GLUT3, compared with the astrocytic glucose transporter, GLUT1 [16]. Lactate is posited to be generated by neurons and taken up by astrocytes. The lactate release model [5] is based on the observed mismatch between total glucose utilization and oxidative metabolism and measured lactate release from brain during brain activation in vivo. If lactate were produced and locally oxidized, total and oxidative metabolism would be similar in magnitude. However, the rise in oxidative metabolism varies with experimental condition and pathways stimulated, it is much less than that of total glucose utilization [5]. Astrocytes have a much faster and greater capacity for lactate uptake from extracellular fluid, and for lactate dispersal among gap junction-coupled astrocytes compared with neuronal lactate uptake and shuttling of lactate to neurons [17]. Astrocytic endfeet surround the vasculature, and can discharge lactate to perivascular fluid for efflux from brain.