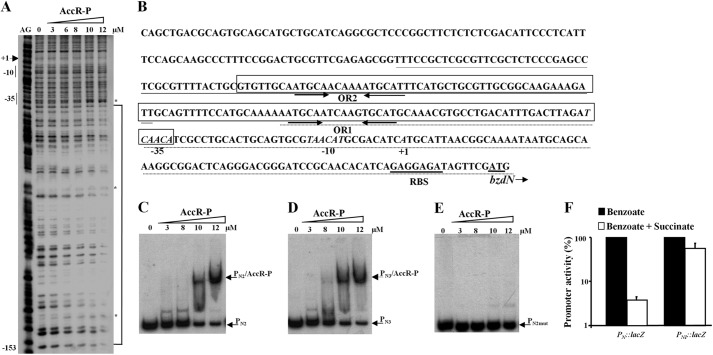
FIGURE 4.
AccR-P binds to two palindromic operator sequences within the PN promoter. A, DNase I footprinting analyses of the PN probe with 0–12 μm carbamoyl phosphate-treated His6-AccR (AccR-P) as described under “Experimental Procedures.” Lane AG shows an A + G Maxam and Gilbert sequencing reaction with the location of the −10 and −35 boxes and the transcription initiation site (+1) indicated to the left. The AccR-P-protected region between positions −153 and −34 is highlighted by a bracket, with phosphodiester bonds mildly hypersensitive to DNase I cleavage indicated by asterisks. B, expanded view of the PN promoter sequence. Inferred −10 and −35 promoter elements and the transcription start site (+1) are indicated in italic type. The ribosome-binding site (RBS) and the bzdN ATG initiation codon are underlined. The region protected by AccR-P (as in A) is boxed with the location of the palindrome operator sequences (OR1 and OR2) indicated by convergent arrows. Nucleotides corresponding to the PN2 and PN3 probes used in C and D are underlined with discontinuous and continuous lines, respectively. C–E, gel retardation analyses of the indicated probes as in Fig. 3B. Gel retardation assays were performed using increasing concentrations (0–12 μm) of carbamoyl phosphate-treated His6-AccR (AccR-P). The free probes and the major DNA·AccR-P complex are indicated by arrows. F, effect of succinate on the activity of the native PN promoter and the truncated PNI promoter that lacks the OR1 and OR2 operators. Azoarcus sp. CIB cells harboring the PN::lacZ translational reporter plasmid pBBR5PN or the PNI::lacZ translational reporter plasmid pBBR5PNI were grown anaerobically in minimal medium containing 3 mm benzoate (black bars) or a mixture of 3 mm benzoate and 0.2% succinate (white bars) until the mid-exponential phase. Values are the average of three independent experiments ± S.D. (error bars) and are graphed setting the β-galactosidase activities obtained for each promoter in the absence of succinate (22,000 and 2,500 Miller units for PN and PNI, respectively) as 100%.