FIGURE 2.
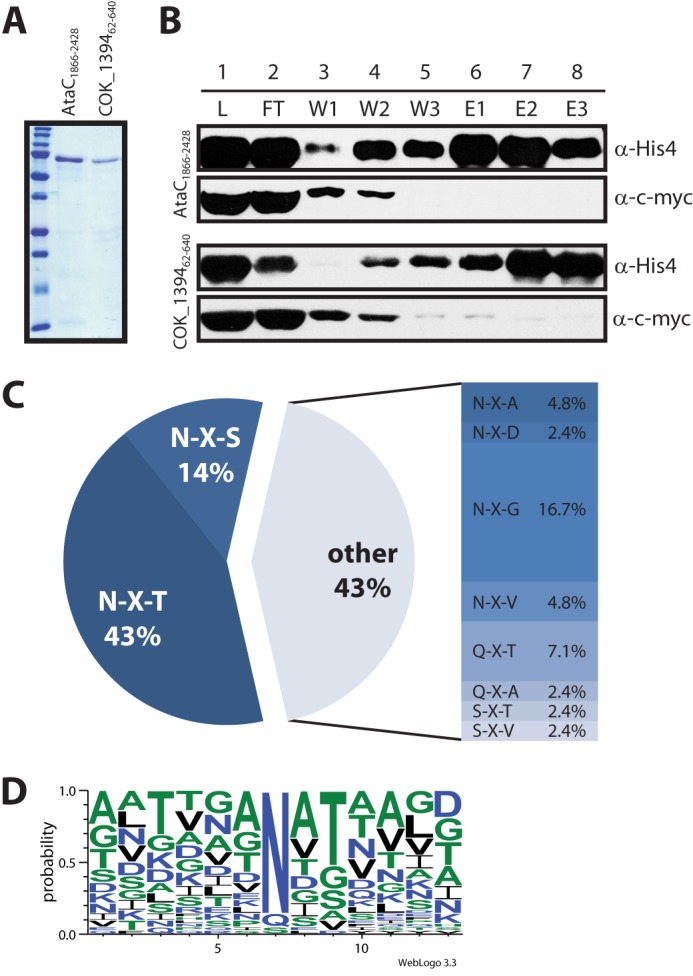
Glycosylation of autotransporter adhesin fragments co-expressed with ApNGT in E. coli. A, purified, glycosylated autotransporter adhesin fragments were analyzed by SDS-PAGE on a 15% acrylamide gel and stained by Coomassie Brilliant Blue R-250. B, fractions from the second purification step (under denaturing conditions) were separated by SDS-PAGE on a 7% acrylamide gel and analyzed by immunoblot. The top two panels show fractions from the purification of AtaC1866–2428, and the bottom two panels show fractions from the purification of COK_139462–640. The autotransporter fragments were detected via the His10 tag, the ApNGT protein via the c-Myc epitope. The fractions analyzed are: load (L, dialyzed eluate from first purification step under native conditions), flow-through (FT), wash fractions (W1–W3, 0, 30, and 40 mm imidazole), and elution fractions (E1–E3, 100 mm imidazole). Note that ApNGT co-purifies with the autotransporter fragments under native conditions (lane 1, L) and is subsequently removed by nickel-nitrilotriacetic acid purification under denaturing conditions. (For details see “Experimental Procedures.”) C, frequency of glycosylated sequons. D, consensus sequence for protein glycosylation by ApNGT in E. coli generated by Weblogo 3.3 (43) from an alignment of all modified sites (42 in total).
