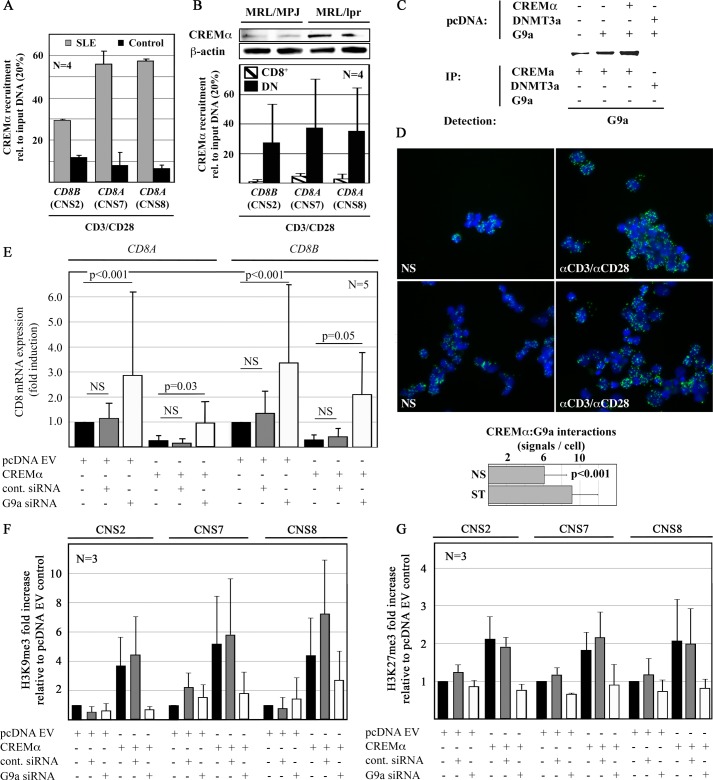
FIGURE 6.
CREMα recruits G9a to the CD8 cluster. A, CREMα recruitment to CNS2, -7, and -8 is enhanced in CD8+ T cells from SLE patients. B, in analogy to SLE T cells, CREMα expression is increased in T cells from MRL/lpr mice (upper panel). In DN but not in CD8+ T cells, CREMα is recruited to CNS2, -7, and -8 suggesting CREMα is involved in the down-regulation of CD8 (lower panel). C, HEK293T cells were transfected with empty pcDNA3.1 plasmids. Proteins were co-immunoprecipitated with anti-CREMα or DNMT3a antibodies as indicated. Lysates were subjected to Western blotting with G9a antibodies. Representative results from one of three independent experiments are displayed. D, an interaction between CREMα and G9a has been established applying PLA. Ex vivo isolated CD8+ T cells exhibit interactions between CREMα and G9a that are enhanced after TCR stimulation (120 h). The number of PLA signals per unstimulated (NS) or stimulated (ST) CD8+ T cell was quantified using ImageJ software. Displayed is the number of signals per CD8+ T cell in 50 visual fields from 5 independent experiments (lower panel). E, G9a knock-down results in an increase of CD8A and CD8B mRNA expression in primary human CD8+ T cells (24 h) when compared with controls siRNA (left, CD8A; right, CD8B, first to third lanes). CREMα reduces CD8A and CD8B mRNA expression (24h) (fourth and fifth lanes). G9a knock-down reverses the CREMα effects on CD8A and CD8B (sixth lane). F and G, CD8A and CD8B expression patterns in response to CREMα with or without G9a knock-down are reflected by histone H3K9 (F) and H3K27 (G) methylation of CNS2, -7, and -8.