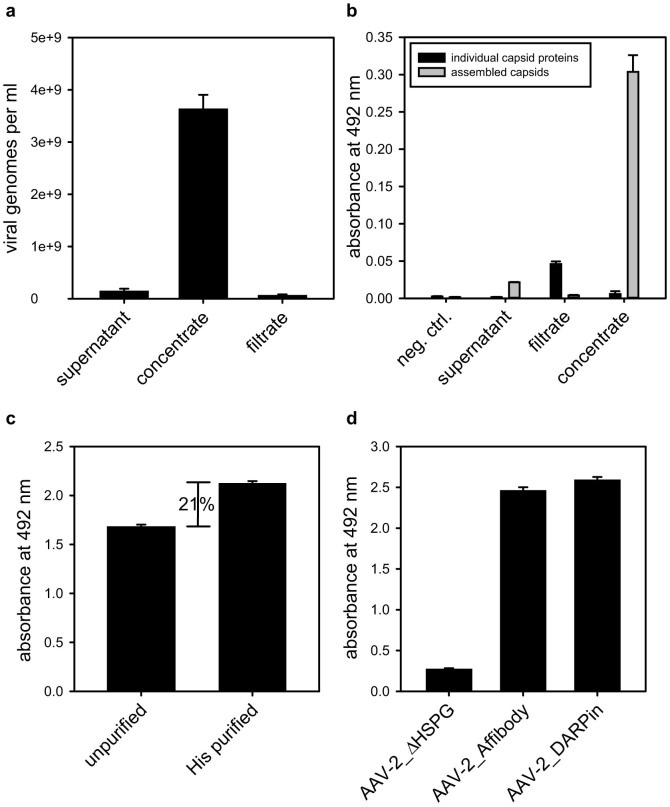
Figure 3. Viral particle concentration, depletion of unassembled capsid proteins, affinity purification and surface display of targeting modules.
(a) Four days post transfection the supernatant from HEK-293 producer cells was harvested (supernatant) and concentrated via centrifugal ultrafiltration (concentrate, filtrate). The number of packed viral particles was determined via qPCR (n = 3, mean ± SD). (b) Samples from viral particle producing HEK-293 cells (supernatant), ultrafiltration filtrate and concentrate and fresh cell medium (neg. control) were incubated on an ELISA plate. Assembled particles or individual capsid proteins were detected using the A20 or the B1 antibody, followed by incubation with HRP-coupled anti-mouse antibody (n = 3, mean ± SD) (c) Ultrafiltration concentrated viral particles were incubated with Ni-NTA resin washed and capsids were eluted with PBS containing 500 mM imidazole. Subsequently, 4 × 109 purified and non-purified particles were incubated in an ELISA plate, coated with anti-His antibody. Viral particles were detected using the capsid specific A20 antibody, followed by HRP-coupled anti-mouse antibody (n = 4, mean ± SD) (d) 1.8 × 109 His-affinity purified AAV-2_AffibodyFlag and AAV-2_DARPinFlag particles were incubated on an ELISA plate coated with anti-Flag antibody. Assembled capsids were detected using the A20 antibody, followed by biotin-coupled isotype specific IgG3 antibody and streptavidin-coupled horseradish peroxidase (n = 3, mean ± SD).