Abstract
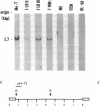
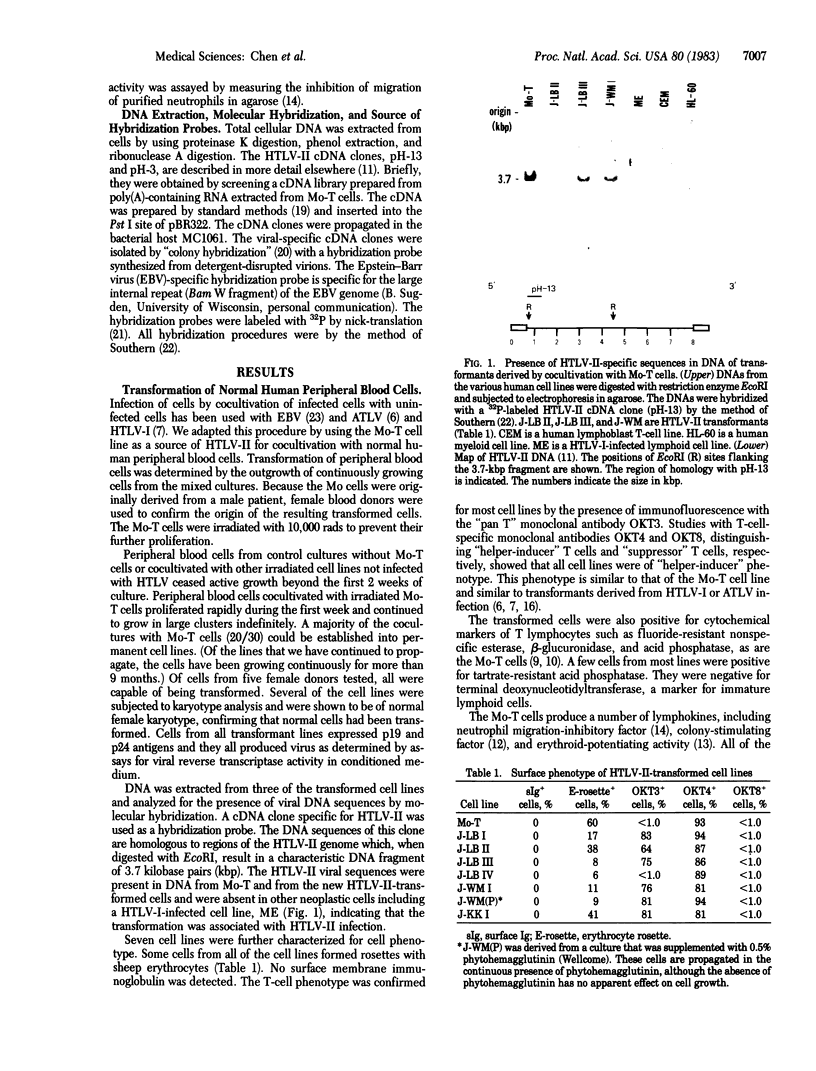
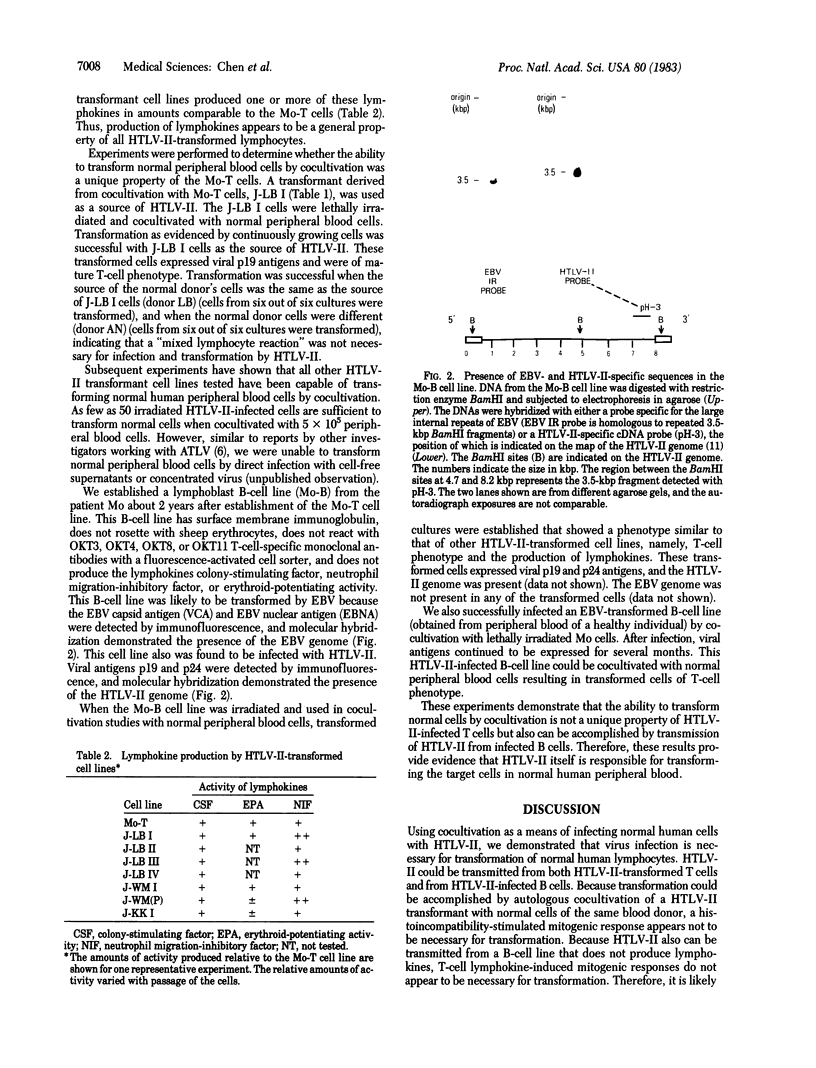
A unique human retrovirus (human T-cell leukemia virus type II, HTLV-II), isolated from a patient with a T-cell variant of hairy-cell leukemia, has been shown to be distinct from the more common isolates of human T-cell leukemia virus. This virus was tested for its ability to transform normal human peripheral blood lymphocytes. The HTLV-II-infected T-cell line Mo-T was lethally x-irradiated and cocultivated with normal human peripheral blood lymphocytes. The cocultivation of normal cells with Mo-T cells resulted in the transformation of the normal cells as evidenced by the establishment of permanent cell lines. The transformed cells are infected with HTLV-II as shown by immunologic tests and molecular hybridization. The cells are of mature T-cell phenotype and constitutively produce lymphokines. An Epstein-Barr virus-transformed lymphoblast B-cell line established from peripheral blood cells of the patient Mo, designated Mo-B, also was found to be infected with HTLV-II. All HTLV-II-infected cells, including the Mo-B cells, were capable of transforming normal cells of T-cell phenotype by transmission of virus by cocultivation. These results indicate that HTLV-II infects both B and T cells but transforms normal human peripheral blood lymphocytes of T-cell phenotype.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Chen I. S., McLaughlin J., Gasson J. C., Clark S. C., Golde D. W. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature. 1983 Oct 6;305(5934):502–505. doi: 10.1038/305502a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Kalyanaraman V. S., Sarngadharan M. G., Sliski A., Vonderheid E. C., Maeda M., Nakao Y., Yamada K., Ito Y., Gutensohn N. Association of the human type C retrovirus with a subset of adult T-cell cancers. Cancer Res. 1983 Aug;43(8):3892–3899. [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Quon D. H., Golde D. W. Purification and characterization of a human T-lymphocyte-derived granulocyte-macrophage colony-stimulating factor. Blood. 1981 Jan;57(1):13–21. [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Reitz M. S., Jr, Sarngadharan M. G., Robert-Guroff M., Kalyanaraman V. S., Nakao Y., Miyoshi I., Minowada J., Yoshida M., Ito Y. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982 Nov 4;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. T-lymphocyte variant of hairy-cell leukemia. Ann Intern Med. 1978 Mar;88(3):323–326. doi: 10.7326/0003-4819-88-3-323. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Quan S. G., Golde D. W. Immunologic characterization of hairy cell leukemias in continuous culture. J Immunol. 1978 Mar;120(3):777–782. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tarella C., Ruscetti F. W., Poiesz B. J., Woods A., Gallo R. C. Factors that affect human hemopoiesis are produced by T-cell growth factor dependent and independent cultured T-cell leukemia-lymphoma cells. Blood. 1982 Jun;59(6):1330–1336. [PubMed] [Google Scholar]
- Weisbart R. H., Lusis A. J., Kacena A., Spolter L., Eggena P., Golde D. W. Neutrophil migration inhibition factor from T lymphocytes (NIF-T): partial purification by antibody affinity chromatography and further characterization. Clin Immunol Immunopathol. 1982 Mar;22(3):408–418. doi: 10.1016/0090-1229(82)90058-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Matsumoto T., Koyanagi Y., Tanaka Y., Hinuma Y. Unique cell lines harbouring both Epstein-Barr virus and adult T-cell leukaemia virus, established from leukaemia patients. Nature. 1982 Sep 23;299(5881):367–369. doi: 10.1038/299367a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]