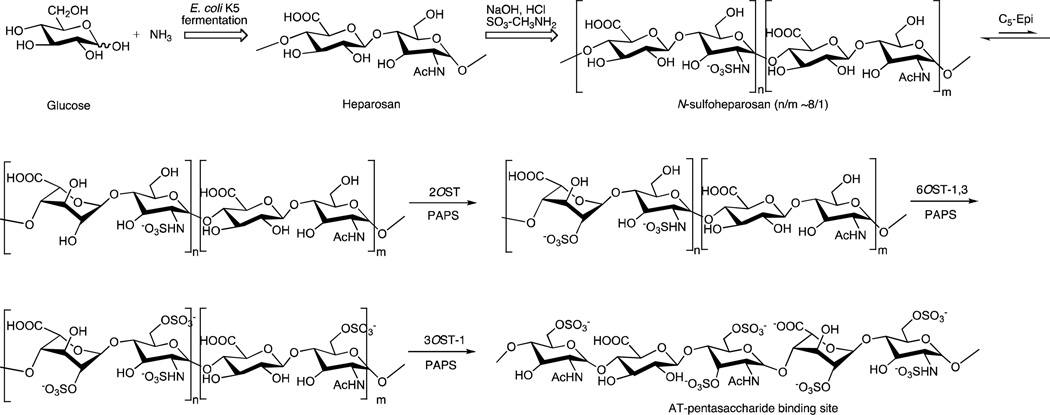
Fig. 1.
Chemoenzymatic synthesis of heparin. Fermentation of E. coli K5 on glucose and ammonia affords heparosan which is then treated with sodium hydroxide to remove over 80% of its N-acetyl groups reduce its molecular weight to afford an N-sulfoheparosan. The N-sulfoheparosan is then converted through a series of enzymatic steps using C5-Epi, 2OST, 6OST-1 and −3 and 3OST-1 to form bioengineered heparin. The structure of an AT-pentasaccharide binding site is shown as the final product. These enzymatic steps closely parallel the final steps within heparin biosynthesis.