Abstract
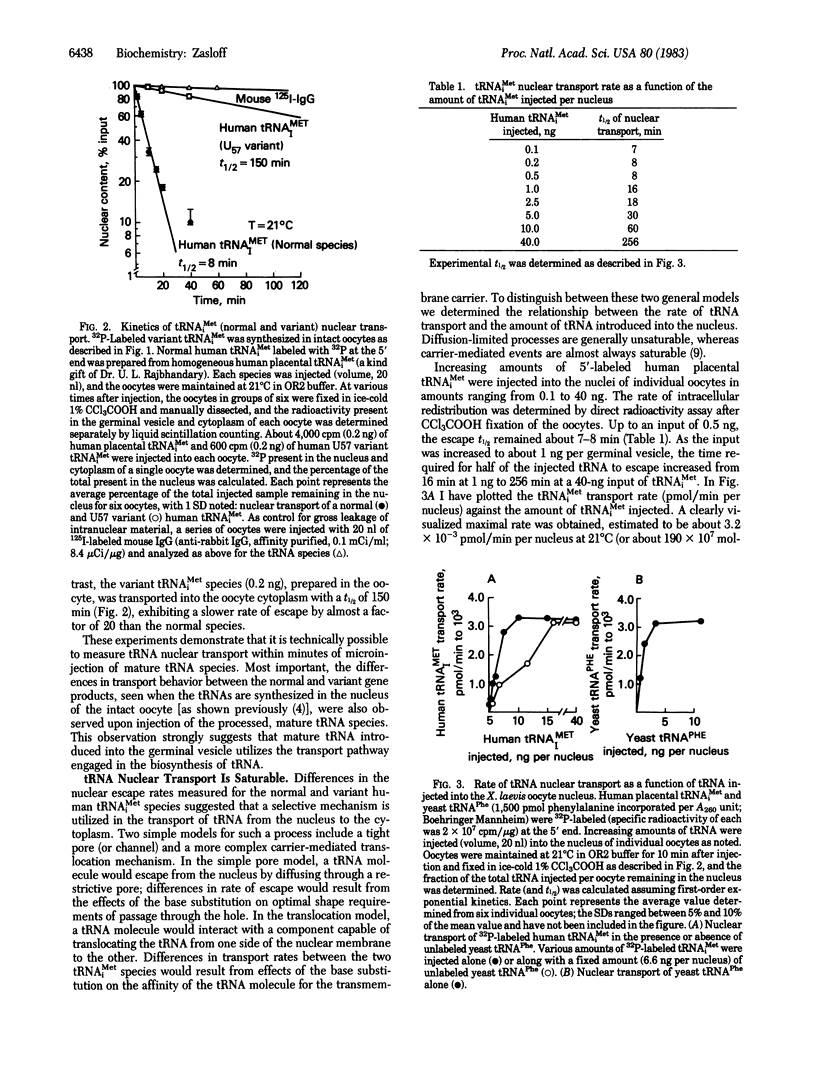
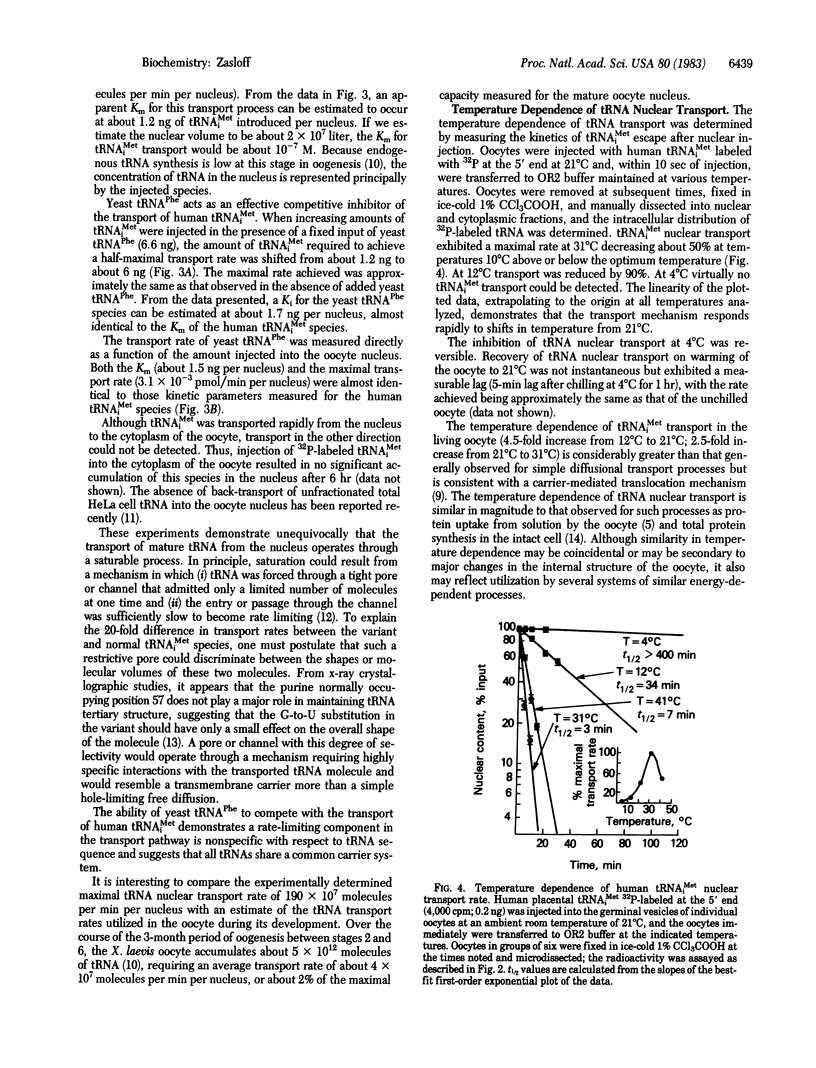
The mechanism by which a tRNA molecule is delivered from the nucleus of a cell to the cytoplasm has been studied in the Xenopus laevis oocyte utilizing nuclear microinjection and manual microdissection techniques. tRNA nuclear transport in this cell resembles a carrier-mediated translocation process rather than diffusion through a simple pore or channel. tRNA transport is saturable by tRNA, with a maximal rate measured to be about 190 X 10(7) molecules per min per nucleus (21 degrees C) in the mature oocyte. Competitive inhibition between two different tRNA species can be demonstrated, suggesting that many tRNA species share a common carrier system. tRNA nuclear transport is sharply dependent on temperature, with an optimal rate observed at 31 degrees C. A single G-to-U substitution at position 57 in the vertebrate tRNAMeti molecule reduces the transport rate of this tRNA by a factor of about 20, implicating this highly conserved region of the tRNA molecule (loop IV) as critical for recognition by the transport mechanism. On morphologic grounds I propose that ribosome-like components surrounding the nuclear pore may function as the tRNA translocation "motor." The tRNA nuclear transport mechanism represents a distinctly eukaryotic process and a site of potential control over cell growth and proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo R., Allende J. E. Conditions affecting protein synthesis in amphibian oocytes. Arch Biochem Biophys. 1976 Feb;172(2):648–653. doi: 10.1016/0003-9861(76)90119-3. [DOI] [PubMed] [Google Scholar]
- Chavancy G., Chevallier A., Fournier A., Garel J. P. Adaptation of iso-tRNA concentration to mRNA codon frequency in the eukaryote cell. Biochimie. 1979;61(1):71–78. doi: 10.1016/s0300-9084(79)80314-4. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Telford J. L., Birnstiel M. L. Transcription of xenopus tDNAmet1 and sea urchin histone DNA injected into the Xenopus oocyte nucleus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1077–1082. doi: 10.1101/sqb.1978.042.01.108. [DOI] [PubMed] [Google Scholar]
- Santos T., Zasloff M. Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNAimet genes. Cell. 1981 Mar;23(3):699–709. doi: 10.1016/0092-8674(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Thomas C. RNA metabolism in previtellogenic oocytes of Xenopus laevis. Dev Biol. 1974 Aug;39(2):191–197. doi: 10.1016/0012-1606(74)90234-6. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wurst R. M., Vournakis J. N., Maxam A. M. Structure mapping of 5'-32P-labeled RNA with S1 nuclease. Biochemistry. 1978 Oct 17;17(21):4493–4499. doi: 10.1021/bi00614a021. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. A model of a poorly-permeable membrane as an alternative to the carrier hypothesis of cell membrane penetration. Bull Johns Hopkins Hosp. 1961 Jul;109:35–48. [PubMed] [Google Scholar]
- Zasloff M., Rosenberg M., Santos T. Impaired nuclear transport of a human variant tRNAiMet. Nature. 1982 Nov 4;300(5887):81–84. doi: 10.1038/300081a0. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Hamer D. H. TRNA precursor transcribed from a mutant human gene inserted into a SV40 vector is processed incorrectly. Nature. 1982 Feb 11;295(5849):533–535. doi: 10.1038/295533a0. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Romeo P., Rosenberg M. Transcription and precursor processing of normal and mutant human tRNAiMet genes in a homologous cell-free system. J Biol Chem. 1982 Jul 10;257(13):7857–7863. [PubMed] [Google Scholar]




