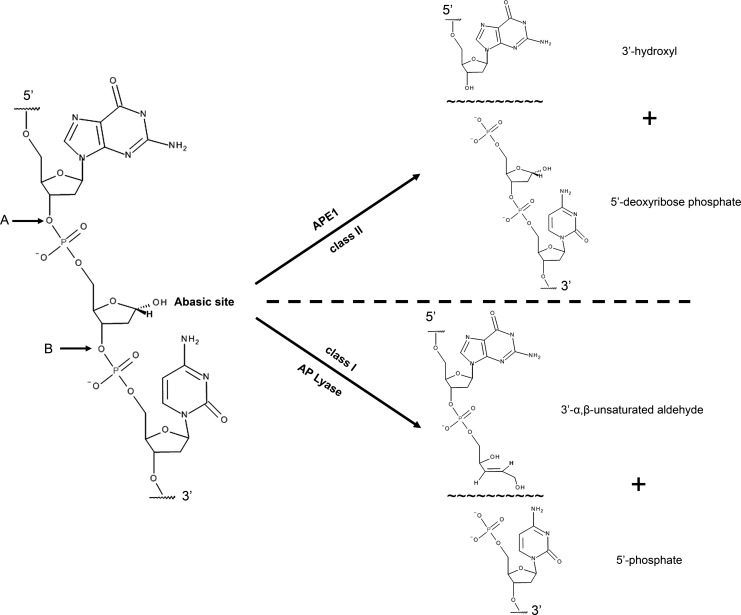
FIG. 1.
Chemical structure of a hydrolytic abasic site and the cleavage position for the major classes of AP site incision enzymes. The phosphodiester bond cleavage site, immediately adjacent to an abasic lesion (see arrows), is shown for a class I AP lyase (site B) and a class II AP endonuclease (site A). Class I AP lyases cleave via β-elimination, generating a 3′-α,β-unsaturated aldehyde and a 5′-phosphate. Class II AP endonucleases, for example, APE1, incise the DNA backbone hydrolytically, leaving behind 5′-deoxyribose phosphate and 3′-hydroxyl termini. For simplicity, just the strand containing the AP site is shown, with two “random” flanking bases. Images were created using the Accelrys Draw 4.1 software (Accelrys, San Diego, CA). AP, apurinic/apyrimidinic; APE1, apurinic/apyrimidinic endonuclease 1.