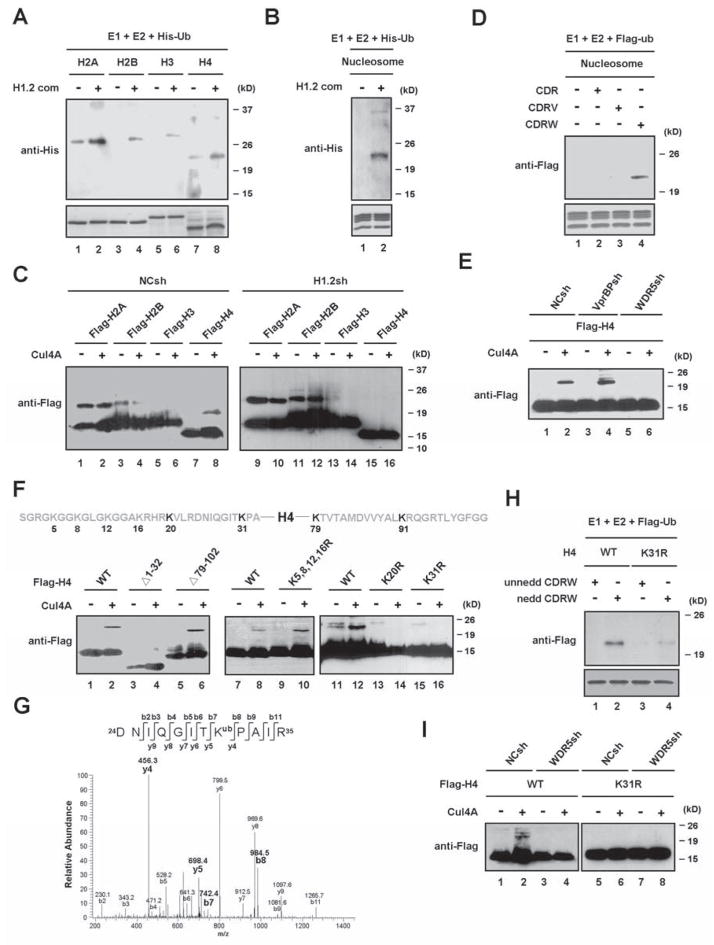
Figure 2. Requirements of H1.2 and WDR5 for Cul4A-mediated H4 ubiquitylation.
(A) The purified H1.2-associated factors were assayed for in vitro ubiquitin ligase activity using individual core histones in the presence of E1, E2 and His-ubiquitin. Reactions were separated on 15% SDS-PAGE and analyzed by immunoblotting with anti-His antibody.
(B) In vitro ubiquitylation assays were performed as in (A), but using HeLa H1-depleted oligonucleosomes as substrates.
(C) Mock- or H1.2-depleted 293T cells were transfected with expression vectors for Flag-core histones and/or Cul4A. 48 h post-transfection, whole cell extracts were prepared and immunoprecipitated with anti-Flag antibody. Levels and the monoubiquitylation status of ectopic histones were determined by immunoblotting with anti-Flag antibody. The mobility-shifted bands correspond to monoubiquitylated histones.
(D) The CDR, CDRV and CDRW complexes containing neddylated Cul4A were assayed for ubiquitin ligase activity using HeLa H1-depleted oligonucleosomes as substrates.
(E) Mock-, VprBP-, or WDR5-depleted cells were transfected with expression vectors for Flag-H4 and/or Myc-Cul4A. Flag-H4 proteins were immunoprecipitated and analyzed by immunoblotting with anti-Flag antibody.
(F) Following the expression of Flag-wild type and mutant H4 and/or Cul4A in 293T cells, the ubiquitylation of ectopic H4 proteins was monitored by immunoblotting as in (C).
(G) Cell lysates from 293T cells transfected with Flag-H4 were immunoprecipitated with anti-Flag antibody and resolved in SDS–PAGE. The band corresponding to ubiquitylated H4 was excised and analyzed by LC–MS/MS mass spectrometry. The MS/MS spectrum shows that the Lys 31 residue is ubiquitylated in the peptide 24-DNIQGITKPAIR-35.
(H) The CDRW complex containing un-neddylated or neddylated Cul4A was assayed for ubiquitin ligase activity using wild type or K31-mutated H4.
(I) Mock- or WDR5-depleted cells were transfected with expression vectors for Cul4A and wild type or mutant H4. Ectopic H4 proteins were immunoprecipitated from whole cell lysates, and their ubiquitylation was probed with anti-Flag antibody. See also Figure S2.