Figure 1.
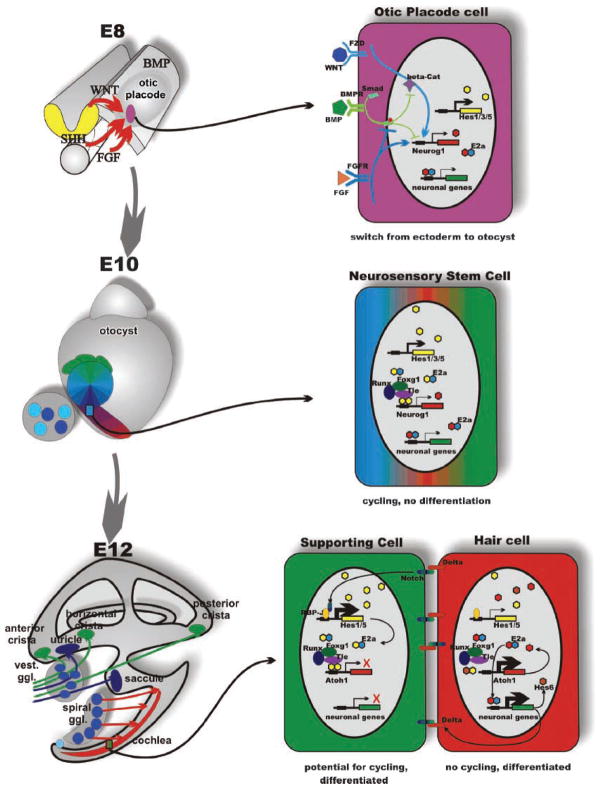
Organ, cell and molecular interactions in ear development. The morphogenesis (left) and some molecular interactions underlying proliferation and cell fate decision (right) are depicted in this scheme. Morphogenesis transforms a small patch of ectoderm between embryonic days 8 and 12 into a complex labyrinth of ducts and recesses that harbors the six sensory epithelia of the mammalian ear in strategic positions for extraction of epithelia-specific energy. Delamination of sensory neurons generates the vestibular and cochlear sensory neurons that connect specific sensory epithelia of the ear to specific targets in the hindbrain. One of the earliest steps in this process is the selection of otic placode cells through the interaction of several diffusible factors; in particular, FGF and WNT signaling upregulates both inhibitory and activating bHLH genes to switch the cell fate through downregulation of BMP signaling, specifying the position and size of the otic placode (top right). These stem cells will, through the interaction of activator- and inhibitor-type bHLH genes remain in cycling phase without differentiation resulting in clonal expansion. As cells progress through the cycles, they will change their fate determination, giving rise to neurosensory stem cells (middle right) that form by asymmetric divisions all sensory neurons of the ear. Some neurosensory stem cells as well as independently arising cells of the otic placode turn into sensory epithelia precursor cells (SNP). These cells will give rise by asymmetric divisions to hair cells and supporting cells (bottom right). Exit from the cell cycle, combined with proper cell fate specification to, eg hair cell and supporting cell, will be mediated in part by the NOTCH-reinforced switch to either explosive upregulation of proneuronal bHLH genes (Atoh1 in the case of hair cells) or of inhibitory bHLH genes (such as Hes1 or Hes5) by the γ-secretase-cleaved Notch fragment that binds to RBPSUH (formerly Rbp-J). The action of HES homodimers on N-boxes to turn on proneuronal genes is enhanced through interaction with the TLE, RUNX, FOXG and genes. Consequently, eliminating for example Foxg1 results in diminished efficacy of HES signaling resulting in premature cell cycle exit and differentiation. Shortly after E14, all proliferative activity in the PNP progenitors stops and no new sensory neurons or hair cells will form. Modified after Refs 37,38.