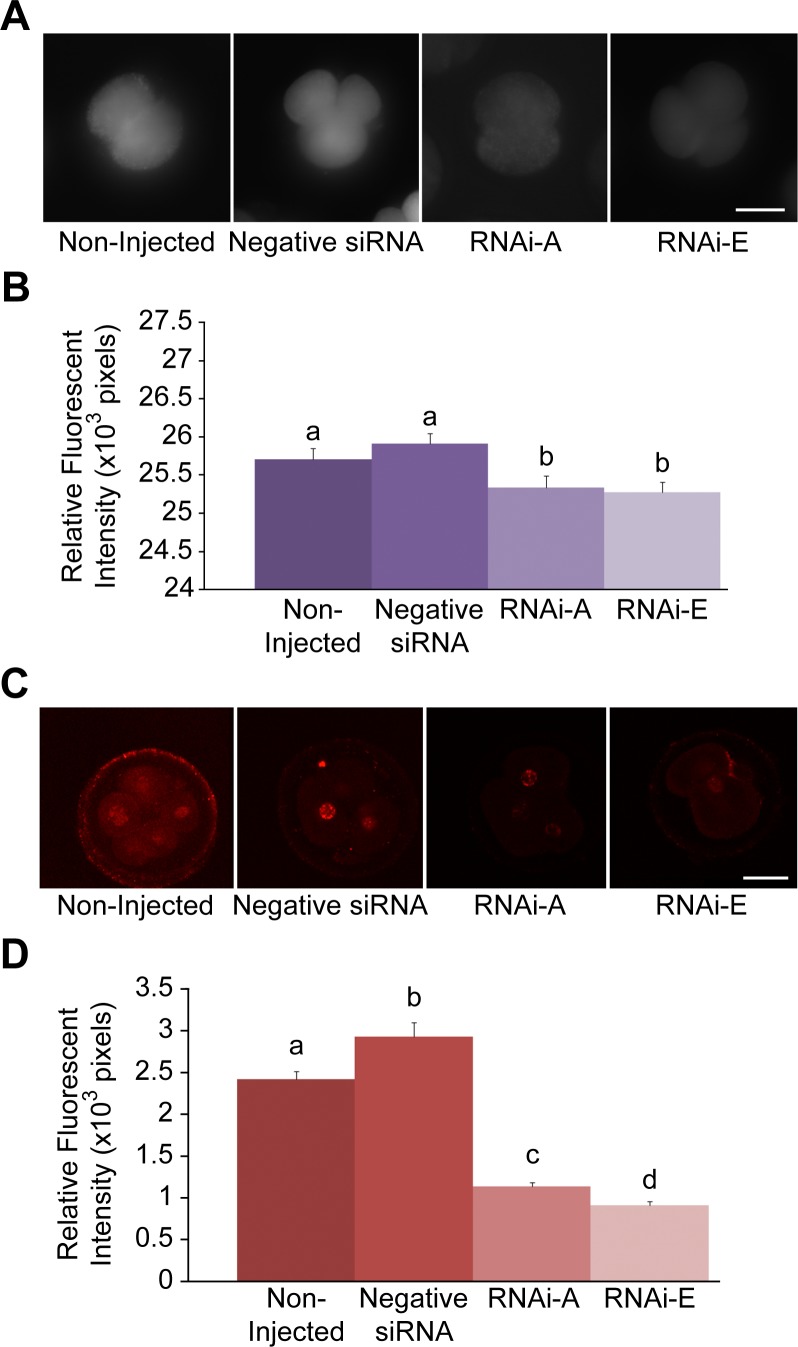
Figure 4. Decreased intracellular ROS and associated DNA damage following RNAi-mediated knockdown of p66Shc in early bovine embryos.
Groups of 2–4 cell embryos were stained with 2′,7′-di- chlorofluorescein (DCF) and immunoassayed for phosphorylated γ-H2A.X protein to measure intracellular reactive oxygen species (ROS) content and DNA damage, respectively. Embryos groups were either non-injected controls, injected with negative control siRNA molecules, or injected with the p66Shc-specific siRNA molecules RNAi-A or RNAi-E. (A) Representative fluorescent images of DCF stained embryos. (B) A slight, non-significant increase in intracellular ROS was observed in negative siRNA-injected embryos over non-injected controls. Embryos injected with p66Shc-specific siRNA molecules exhibited significantly (P<0.05) reduced ROS levels. Different letters above the histogram bars represent significant differences (P<0.05) in intracellular ROS intensities. There were no significant difference between RNAi-A and RNAi-E injected embryos observed. (C & D) Quantification of ROS-associated DNA damage following RNAi-mediated p66Shc knockdown. Groups of 2–4 cell embryos were stained for phosphorylated γ-H2A.X protein for quantification by relative immunofluorescent signal intensities. Embryos were: non-injected (controls), injected with negative control siRNA molecule, or injected with the p66Shc-specific siRNA molecules RNAi-A or RNAi-E. (C) Representative fluorescent images of phospho-γ-H2A.X stained embryos. (D) Negative siRNAinjected embryos exhibited significantly higher levels of phosphorylated γ-H2A.X protein than non-injected controls. Embryos injected with either RNAi-A or RNAi-E molecules exhibited a significant decrease in phosphorylated γ-H2A.X signal intensities compared to controls. Different letters above the histogram bars represent significant differences (P<0.05) in mean phospho-γ-H2A.X fluorescent intensities. Scale bar, 50 µM.