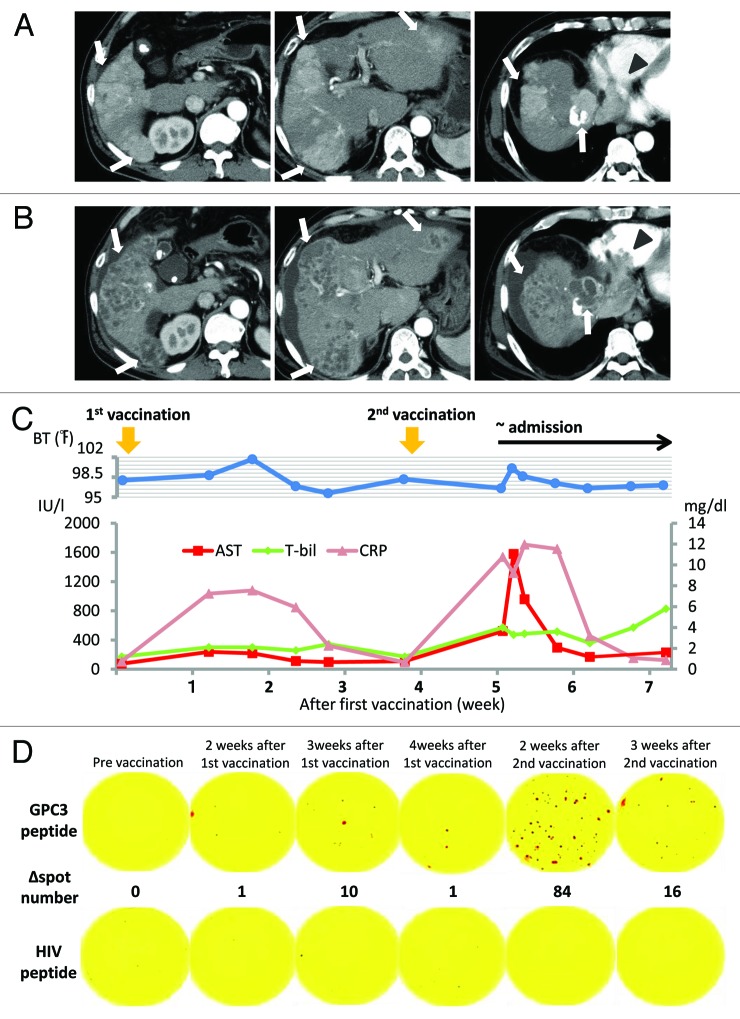
Figure 1. Findings of an early-phase contrast-enhanced CT (CT) scan. (A) Contrast-enhanced CT scan before vaccination shows a 68 × 51-mm tumor with multiple intrahepatic tumors (arrow) and a 44 × 30-mm tumor invading the right atrium (arrowhead). (B) Contrast-enhanced CT after the second vaccination showing multiple low-density areas in the liver, indicating extensive tumor necrosis (arrow). By contrast, a tumor thrombus in the right atrium increased to a 83 × 50-mm tumor (arrowhead). (C) Clinical course from the beginning of GPC3 peptide vaccination. Approximately 1 week after the first vaccination, the patient began reporting general fatigue and showed intermittent fever. Inflammatory and hepatic parameters were elevated (CRP: pink line, AST: red line, T-bil: green line). The abnormal laboratory parameters improved after observation. On day 9 after the second vaccination, the patient was admitted to our hospital as an emergency due to fever and general fatigue, which were similar to his previous symptoms. One day after hospitalization, the inflammatory and hepatic parameters were remarkable. Inflammatory and hepatic parameters improved 1 week after hospitalization. However, his status gradually worsened, and he died on day 30 after the second vaccination. (D) Immunological monitoring of the GPC3 peptide-specific T cell responses. Ex vivo IFN-γ enzyme-linked immunospot (ELISPOT) assays against GPC3 in 5 × 105 peripheral blood mononuclear cells (PBMCs) were performed before and after vaccination. The ∆ spot number indicates the number of GPC3 peptide-specific cytotoxic T-lymphocytes (CTLs). The number of interferon (IFN)-γ positive spots increased from 0 to 84 after the second vaccination.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.