Figure 10. Strategy of NSM-functionalization of drug carriers to bypass carrier size-restrictions and improve endocytosis.
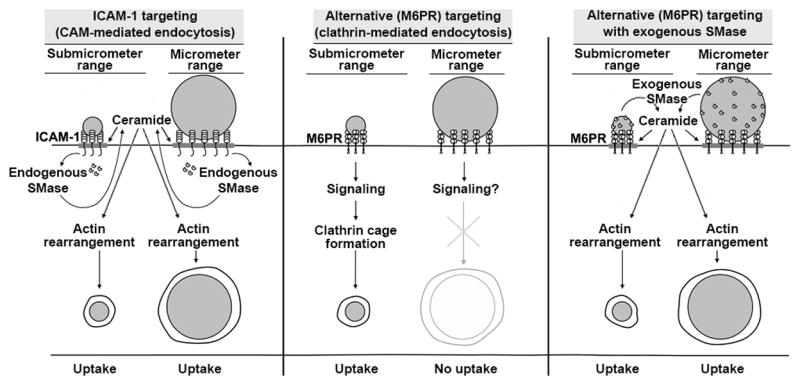
ICAM-1-targeted nano- and micro-carriers are both efficiently internalized by cells due to sphingomyelinase (SMase)-dependent generation of ceramide at carrier-binding sites on the plasmalemma, which is associated with CAM-mediated endocytosis (left panel). Ceramide improves carrier engulfment and membrane invagination, and acts as a second messenger toward actin re-organization, helping in endocytosis. In contrast, targeting drug carriers to receptors associated with more size-restrictive pathways, e.g., clathrin-associated M6PR, often enables intracellular transport of nano- but not micro-carriers (middle panel). Surface-functionalization of said carriers with elements mimicking the CAM-mediated pathway, namely exogenous SMases (such as NSM in this study), does not impact binding but supplies ceramide and actin re-organization, improving endocytosis of nano- and micro-carriers even when targeted to receptors different from ICAM-1 (right panel).
